Abstract
Background
We aimed to clarify the long-term outcomes of patients with T1 colorectal carcinoma (CRC) after endoscopic resection (ER) and surgical resection.
Methods
We examined T1 CRC patients treated during 1992–2008 and who had ≥5 years of follow-up. Patients who did not meet the curative criteria after ER according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines were defined as “non-endoscopically curable” and classified into three groups: ER alone (Group A: 121 patients), additional surgery after ER (Group B: 238 patients), and surgical resection alone (Group C: 342 patients). Long-term outcomes and predictors of recurrence were analyzed.
Results
Of the 882 patients with T1 CRC, 701 were non-endoscopically curable. Among these patients, recurrence and 5-year overall survival (OS) rates were 0.6 and 91.1%, respectively. In Groups A, B, and C, recurrence rates were 5.0, 5.5, and 3.8%, OS rates were 79.3, 92.4, and 91.5% (p < 0.01), and 5-year disease-free survival (DFS) rates were 98.1, 97.9, and 98.5%, respectively. Thirty-two patients experienced local recurrence or distant/lymph node metastasis (Group A: 6; Group B: 13; Group C: 13) and 14 patients died of primary CRC (Group A: 3; Group B: 7; Group C: 4). Age ≥65 years, protruded gross type, positive lymphatic invasion, and high budding grade were significant predictors of recurrence in non-endoscopically curable patients.
Conclusions
Our findings supported the JSCCR criteria for endoscopically curable T1 CRC. ER for T1 CRC did not worsen the clinical outcomes of patients who required additional surgical resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) has the greatest or second-greatest incidence of all carcinomas in Western countries and Japan [1, 2]. It has been reported that patients with intramucosal CRC do not develop lymph node (LN) metastasis and are, therefore, good candidates for endoscopic resection (ER) [3]. In Japan, endoscopic submucosal dissection (ESD) is regarded as a reliable method for en bloc resection, regardless of tumor size as total excisional biopsy in cases of clinical T1 CRC [4–6]. The frequency of cases in which ER is applied to T1 CRC has increased in parallel with the aging of the Japanese population [7]. The policy for treating pathological T1 CRC after ER follows the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014. In these guidelines, the curative criteria for T1 CRC after ER are well/moderately differentiated or papillary carcinoma, no vessel invasion, submucosal invasion depth <1000 μm, and budding grade 1, because of the very low risk of LN metastasis [8]. However, the JSCCR criteria were established based on the analysis of histologic data on T1 CRC from surgically resected specimens [9]. Approximately 90% of patients with T1 CRC do not have LN metastasis [8–17], thus subsequent surgery may amount to overtreatment. Recently, many reports have investigated stratifications of LN metastasis in T1 CRC [9, 12, 13, 16, 18–21]. Various studies have been conducted on the criteria for surgical indication, with the twin aims of identifying high-risk patients (thereby preventing unfavorable outcomes) and decreasing the incidence of unnecessary surgery [9, 12, 13, 16].
Some reports have considered the prognosis of T1 CRC after treatment [15, 18, 22–25]. However, these reports had short mean follow-up periods after treatment and included a relatively small number of cases. In addition, there have been no studies in which pathologists reevaluated each pathological specimen in detail. Moreover, the characteristics of recurrence after ER have not been reported. The aim of this study was to analyze the long-term outcomes of patients with T1 CRC after treatment, including surgical resection alone. The following outcomes were evaluated: recurrence, 5-year disease-free survival (DFS), 5-year disease-specific survival (DSS), and 5-year overall survival (OS) rates.
Methods
Patients
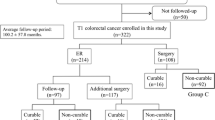
This study enrolled 1143 patients with T1 CRC treated between January 1992 and December 2008 at Hiroshima University Hospital and 10 affiliated hospitals (Hiroshima GI Endoscopy Research Group) and followed up for ≥5 years (Fig. 1). Patients with previous or synchronous CRC, familial adenomatous polyposis, inflammatory bowel disease, or a follow-up period of <5 years were excluded. Patients who underwent surgical resection without LN dissection (transanal endoscopic microsurgery or local resection) as initial treatment for T1 CRC were also excluded. Patients who were diagnosed as curable T1 CRC after en bloc ER according to the JSCCR criteria were defined as “endoscopically curable (e-curable)” patients. Those who did not meet the criteria were defined as “non-e-curable” patients. Moreover, non-e-curable patients were classified into three groups according to the treatment method: Group A included patients who underwent ER alone, Group B included patients who underwent ER and additional surgical resection with LN dissection, and Group C included patients who underwent surgical resection with LN dissection alone. Group A patients were followed despite their non-e-curable status; they were not candidates for additional surgery because of anesthetic concerns (e.g., synchronous carcinoma in another organ, cardiovascular disease, respiratory disease, renal failure, or liver dysfunction), advanced age, or both. Group B patients underwent radical resection (e.g., bowel resection) and regional LN dissection without delay after surgery became indicated. The study protocol was approved by the ethics committee of Hiroshima University (937) and each affiliated hospital and was in keeping with the guidelines of the relevant government agency. All patients gave their informed consent before all procedures.
Indication and procedure of ER
According to the latest JSCCR 2014 guidelines [8], the indication criteria for ER are as follows: (1) intramucosal carcinoma or carcinoma with superficial submucosal invasion, (2) size irrelevant, and (3) any macroscopic type [8]. The methods of ER included polypectomy, endoscopic mucosal resection (EMR), and ESD. Patients who enrolled in this study were treated according to the Japanese Classification of Colorectal Carcinoma (from January 1992 to May 2005) [26–28] or the JSCCR 2005 guidelines (from June 2005 to December 2008) [29] at that time. Therefore, there were some differences in criteria between the JSCCR 2014 guidelines and the above ones.
Indication of additional surgical treatment
The JSCCR 2014 guidelines state that a positive deep tumor margin is an absolute indication for additional surgery after ER [8]. Additional surgical treatment should be considered when at least one of the following is found: (1) submucosal invasion depth ≥1000 μm; (2) positive vessel invasion; (3) poorly differentiated adenocarcinoma, signet ring cell carcinoma, or mucinous carcinoma; or (4) budding grade 2/3 at the deepest part of submucosal invasion [8]. A budding is defined as a single cancer cell or a cluster of <5 cells along the invasion margin, and budding is graded per microscopic field at 200× magnification (i.e., grade 1: 0–4 buds; grade 2: 5–9 buds; grade 3: ≥10 buds) [16]. Budding grade 2/3 is defined as high grade, and grade 1 as low grade. The JSCCR guidelines clearly state that additional surgical treatment should be performed only after systematically evaluating the predicted curability based on various LN metastasis risk factors and the patient’s condition (age, physical performance, presence of adverse events, etc.), and after obtaining informed consent from the patients.
Histologic examination
Histologic diagnosis was performed after ER or surgical resection, and measurements of submucosal invasion depth were obtained according to the JSCCR 2014 guidelines [8]. In all cases, experienced gastrointestinal pathologists (F. S. or K. K.) reevaluated each pathologic specimen for resection margin status and tumor characteristics, including histologic type, depth of submucosal invasion, lymphatic invasion, vessel invasion, and tumor budding regardless of the previous diagnosis that had been made at each of the institutions.
Surveillance schedule after treatment
Physical examinations, chest radiography, contrast enhanced computed tomography of the abdomen and pelvis, and blood tests (including carcino-embryonic antigen level) were performed every 6 months postoperatively for the first 3 years, and thereafter every 12 months in principle. An annual total colonoscopy was performed. Confirmation of recurrence was based on imaging and/or pathological findings. Local recurrence was defined as recurrence at the site of resected CRC in the case of ER, or within the surgical field of colonic carcinoma or within the pelvis for rectal carcinoma in the case of surgical resection. Distant recurrence was defined as the occurrence of metastasis of colorectal origin associated with the index tumor. To detect recurrence after treatment, a follow-up examination period of at least 5 years was set.
Investigated variables
We compared various demographic and clinical characteristics among Groups A, B, and C: age, gender, the presence of malignant diseases in other organs, tumor location, tumor size, gross type, the presence of adenomatous component, histologic type, submucosal invasion depth, vertical margin, lymphatic invasion, venous invasion, budding grade, and lymph node metastasis. Long-term outcomes were evaluated in each group. The clinical outcomes of recurrence rate, OS rate, DFS rate, and DSS rate were assessed. OS assessed time to death from any cause, while DFS was defined as freedom from confirmed recurrence or death from T1 CRC, and DSS was defined as time to death from T1 CRC. Moreover, we analyzed predictors that had significant associations with recurrence using Cox regression analysis.
Statistical analysis
Values are reported as means (standard deviations). Fisher’s exact test was used to compare categorical variables. Analyses were performed with JMP Statistical software version 10.0.2 (SAS Institute, Cary, NC, USA). p values <0.05 were considered statistically significant. The OS, DFS, and DSS rates were calculated using the Kaplan–Meier method. Cox regression analysis was used to calculate hazard ratios for recurrence for the following variables: age, gender, resection method, location, tumor size, the presence of adenomatous component, gross type, histologic type, submucosal invasion depth, lymphatic invasion, venous invasion, and budding grade.
Results
Clinicopathological characteristics of T1 CRC
We evaluated 882 patients with T1 CRC [e-curable; 181 patients, non-e-curable; 701 patients, mean follow-up; 100.8 ± 46.8 months (median 92.0 months), Group A:B:C 86.6 ± 46.7:104.0 ± 46.6:104.5 ± 47.7 months]. The demographic and clinical characteristics of the patients classified as non-e-curable (Group A: 121 patients, male/female 79/42 patients; Group B: 238 patients, male/female 149/89 patients, and Group C: 342 patients, male/female 187/155 patients) are shown in Table 1. The mean age in Group A (69.3 ± 10.7 years old) was significantly higher than that in Group B (63.3 ± 10.7 years old) and C (66.1 ± 10.1 years old) (p < 0.01). There were no significant differences in tumor location among the three groups. The average tumor size in Group C (20.3 ± 11.2 mm) was larger than that in Group B (18.3 ± 11.6 mm) (p < 0.05). Regarding the gross type, the incidence of superficial type in Group C (51.2%, 175/342) was significantly higher than those in Groups A (19.8%, 24/121) and B (15.1%, 38/238) (p < 0.01). The incidences of the adenomatous component in Groups A (69.4%, 84/121) and B (64.7%, 154/238) were higher than that in Group C (31.6%, 108/342) (p < 0.01). There were no significant differences in histological grade among the three groups. The incidence of submucosal invasion depth <1000 μm in Group A (17.4%, 21/121) was significantly higher than those in Groups B (8.0%, 19/238) and C (5.9%, 20/342) (p < 0.01). The incidence of lymphatic invasion in Group B was significantly higher than that in Group A (37.0 vs. 25.6%, p < 0.05). Additionally, the incidence of lymphatic invasion in Group C was higher than that in Group B (59.7 vs. 37.0%, p < 0.01). There were no significant differences in budding grade or LN metastasis among the three groups.
Recurrence after treatment for T1 CRC
Among the e-curable patients with T1 CRC, the recurrence, OS, DFS, and DSS rates were 0.6% (1/181), 91.1, 99.4, and 99.4%, respectively (Fig. 2). Only one case of recurrence was observed in the surgery group, and the patient died of T1 CRC. The recurrence and mortality rates in the three groups (Groups A, B, and C) are shown in Table 2. The overall recurrence rate was 4.6% (32/701), while those in Groups A, B, and C were 5.0% (6/121), 5.5% (13/238), and 3.8% (13/342), respectively. There was no significant difference in overall recurrence rate between the subgroups. The overall mortality rate was 19% (133/701). The mortality rate in Group A (31%, 38/121) was significantly higher than those in Groups B (16%, 38/238) and C (17%, 57/342) (p < 0.01). The overall mortality rate associated with T1 CRC was 2.0% (14/701), while those in Groups A, B, and C were 2.5% (3/121), 2.9% (7/238), and 1.2% (4/342), respectively. There was no significant difference between the subgroups.
Kaplan–Meier curves for a overall survival, b disease-free survival, and c disease-specific survival rates in the endoscopically curable (e-curable) group (n = 181). a Overall survival rate (91.1%). b Disease-free survival rate (99.4%). c Disease-specific survival rate (99.4%). Key: red line patients who underwent endoscopic resection (ER) alone in the e-curable group; green line patients who underwent ER and additional surgical resection with lymph node dissection in the e-curable group; blue line patients who underwent surgical resection with lymph node dissection alone in the e-curable group. ER endoscopic resection
The OS rates were 79.3, 92.4, 91.5% in Groups A, B, and C, respectively. The OS rate in Group A was significantly lower than those in Groups B and C (p < 0.01) (Fig. 3). The DFS rates were 98.1, 97.9, 98.5% in Groups A, B, and C, respectively, and the corresponding DSS rates were 99.1, 98.3, and 99.1%, respectively. Local recurrence or distant metastasis was observed in 32 patients (Group A: 6; Group B: 13; Group C: 13). Four (3.3%, 4/121), 6 (2.5%, 6/238), and 2 (0.6%, 2/342) patients had local recurrences in Groups A, B, and C, respectively. There was significant difference in local recurrence between Groups A and C (p < 0.05); however, no significant difference was observed between Group A and Groups B/C (in which surgical resection was performed).
Kaplan–Meier curves for a overall survival, b disease-free survival, and c disease-specific survival rates in the non-endoscopically curable (non-e-curable) group (n = 701). a Overall survival rate, b disease-free survival rate, c disease-specific survival rate. Key: red line Group A; green line Group B; blue line Group C
Table 3 summarizes the details of the cases with recurrence in Groups A, B, and C. In Group A, all patients had risks of LN metastasis; positive vessel invasion, and/or high budding grade, regardless of submucosal invasion depth. In Group B, all of the patients except one had positive vessel invasion and/or high budding grade, regardless of submucosal invasion depth. In Group C, all patients had positive vessel invasion and/or high budding grade, regardless of submucosal invasion depth. For lesions that were well/moderately differentiated or papillary adenocarcinomas that lacked vessel invasion and had grade 1 tumor budding, the incidence of recurrence was 0.4%, even in cases with relatively deep submucosal invasion depth.
The results of the Cox regression analysis of DFS are shown in Table 4, as summarized for all groups. Age ≥65 years old [hazard ratio 2.35; 95% confidence interval (CI) 1.10–5.38; p = 0.03], protruded gross type (hazard ratio 5.73; 95% CI 1.95–24.5; p < 0.01), positive lymphatic invasion (hazard ratio 2.80; 95% CI 1.29–6.61; p < 0.01), and high budding grade (hazard ratio 2.82; 95% CI 1.32–5.86; p < 0.01) were significant predictors of recurrence after treatment for T1 CRC. On the other hand, gender, resection method, tumor location, tumor size, the presence of an adenomatous component, histology, submucosal invasion depth, and venous invasion were not significant predictors of recurrence in the multivariate analysis.
Discussion
This is the first retrospective multicenter cohort study to have examined the long-term outcomes of patients with T1 CRC according to the latest JSCCR guidelines. Previous studies of T1 CRC treatment outcomes have been subject to important limitations: short follow-up periods and a general lack of data regarding pathological findings, including tumor budding [19, 22, 24]. We reported that regardless of submucosal invasion depth, the LN metastasis rate was approximately 1.2% for cases of T1 CRC in which the following features were not detected: vessel invasion, poorly differentiated adenocarcinoma, signet ring cell carcinoma, mucinous carcinoma, or grade 2/3 budding at the deepest part of submucosal invasion [13]. We concluded that ER without additional surgical resection was valid for cases of well- or moderately differentiated adenocarcinoma with a depth of submucosal invasion <1000 μm and no lymphatic or venous involvement [19]. Kitajima et al. [9] reported that submucosal invasion depth, lymphatic invasion, and tumor budding are risk factors for LN metastasis. In addition, the condition of the muscularis mucosae was an indicator of LN metastasis [20, 21]. Only 1.9% of low-risk patients had LN metastasis, regardless of submucosal invasion depth [18]. It was reported that recurrence without LN metastasis occurs in 0–3.7% of surgically resected pT1 CRCs [30–32]. However, these reports were based on the small number of patients. Kobayashi et al. [15] reported that recurrence occurred in 2.3% of patients with T1 CRCs over a median follow-up period of 7.8 years, even if surgery with LN dissection had been performed. Our data showed that the incidence of recurrence was 3.8% in the group of patients who underwent surgical resection with LN dissection alone.
A few studies have evaluated the beneficial effect of additional surgery after ER as an oncological outcome in T1 CRC. Choi et al. [33] reported that approximately 16% of high-risk T1 CRC patients benefited from subsequent additional surgery because of LN metastasis or recurrence. Among 30 patients in their study who did not have risk factors for LN metastasis, none subsequently developed LN metastasis or recurrent CRC. Yoshii et al. [18] reported that, among patients with high-risk T1 CRC, the cumulative risk of recurrence for patients who underwent additional surgery after ER was 3.7%, which was significantly lower than that for patients who underwent ER alone (20.1%). They concluded that additional surgery after ER was recommended for patients with high-risk T1 CRC. In our study, there were no significant differences in DFS rates between patients with ER alone and patients who underwent additional surgery with LN dissection after ER. Rickert et al. [34] reported that ER for malignant polyps did not worsen surgical and oncologic outcomes in patients who underwent an additional surgery after ER and suggested that oncologic resection for residual tumors should be undergone. Also, it has been reported that the risks of LN metastasis and recurrence for T1 CRC after secondary surgery (ER was performed first) compared with primary surgery did not increase [35]. Further studies are needed to clarify these clinical issues.
Overall, local recurrence rates of 0–11% have been reported for T1 CRC after ER [16, 18, 22–25, 33]. Ueno et al. [16] reported that the incidence of local recurrence was 4.9% (2/41) with local excision alone, and all two patients with local recurrence had risk factors for recurrence. Choi et al. [33] reported local recurrences in three of 42 patients (7.1%) who did not receive additional surgery after ER, as compared with no recurrence in 45 patients who received an additional surgery after ER. In this study, local recurrence occurred in four patients with T1 CRC from among the 121 patients (3.3%) treated with ER alone and these four patients had more than one risk factor for LN metastasis. Our data suggests that additional surgery with LN dissection after ER should be considered for patients with T1 CRC who have risk factors for LN metastasis, according to the JSCCR guidelines.
It has been reported that approximately 90% of recurrences after curative resection of CRC occur within 5 years after surgery [36]. Our data showed that more than 90% of recurrences were detected within 5 years after treatment, although recurrence was detected more than 80 months after treatment in two cases (one case with ER alone, and the other case with surgical resection alone). Therefore, the recommended duration of follow-up after treatment of T1 CRC may be approximately 10 years. In the previous studies, the interval of time until recurrence after treatment was within 5 years; therefore, after the treatment of T1 CRC, follow-up examinations are required for at least 5 years or longer [22, 24]. Our data showed that the group of patients treated with ER alone included non-e-curable patients who did not undergo surgery because of anesthesia concerns (e.g. concomitant diseases), refusal to receive additional surgery and/or advanced age (over 80 years old). This is one of the reasons why the OS rate of the group of patients treated with ER alone was significantly lower than the other groups. Patients’ backgrounds were taken into consideration sufficiently. Even when treatment is performed following the JSCCR guidelines 2014 for T1 CRC, patients are still surgically overtreated. In the near future, immunohistochemical analyses of molecular markers at the site of the deepest penetration of the T1 CRC specimen may allow LN metastasis to be predicted, regardless of the present standard pathologic risk factors that are identified with only hematoxylin and eosin staining [37].
It has been reported that patients whose only risk factor is deep submucosal invasion have a low cumulative risk of recurrence without surgery [18, 23]. In the present study, the incidence of recurrence was only 0.4%; there was only one case of recurrence among patients who met three of the four JSCCR curative criteria for T1 CRC after ER. We anticipate that the indication of ER for clinical T1 CRC (even with a deep submucosal depth of invasion) will be expanded as total excisional biopsy, especially for elderly patients with concomitant disease. When deciding upon additional surgery after ER, endoscopists should consider the individual patient’s age, concomitant diseases, wishes, life expectancy, performance status, and concrete risk of LN metastasis, as well as the operative method. The surveillance method in this study is thought to be adequate from our results. However, a further prospective study is necessary to reveal an appropriate surveillance program for the patients with “non-e-curable” after ER for T1 CRC. Ikematsu et al. [22] reported that the tumor location (rectum) was a significant contributor to recurrence after treatment for T1 CRC with ER alone in a high-risk group. Our results revealed that the significant predictors of recurrence after treatment for T1 CRC included age ≥65 years old, protruded type, positive lymphatic invasion, and high budding grade. It would be beneficial for us to evaluate these risk factors prospectively in the near future, and it is necessary to suggest a stricter surveillance program after treatment for the patients with above four factors.
This study has some limitations. First, this study was a retrospective analysis based on the clinical records. Second, this study might have been affected by selection bias because it was not randomized. Third, the statistical power was not sufficient to discern small differences in subgroup analyses of more comprehensive pathological factors.
In conclusion, the long-term outcomes supported the JSCCR 2014 criteria for e-curable T1 CRC. Recurrence occurs even in patients with T1 CRC who undergo surgical resection. ER did not worsen the clinical outcomes of patients who required additional surgical resection of T1 CRC. We expect that the indication of ER for T1 CRC (even with deep submucosal invasion) will be expanded.
References
Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81.
Matsuda T, Marugame T, Kamo K, et al. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2012;42:139–47.
Morson BC, Whiteway JE, Jones EA, et al. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut. 1984;25:437–44.
Asayama N, Oka S, Tanaka S, et al. Endoscopic submucosal dissection as total excisional biopsy for clinical T1 colorectal carcinoma. Digestion. 2015;91:64–9.
Ozawa S, Tanaka S, Hayashi N, et al. Risk factors for vertical incomplete resection in endoscopic submucosal dissection as total excisional biopsy for submucosal invasive colorectal carcinoma. Int J Colorectal Dis. 2013;28:1247–56.
Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697–707.
Saitoh Y, Inaba Y, Sasaki T, et al. Management of colorectal T1 carcinoma treated by endoscopic resection. Dig Endosc. 2016;28:324–9.
Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for the treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–39.
Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–43.
Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–6.
Hassan C, Zullo A, Risio M, et al. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48:1588–96.
Yasuda K, Inomata M, Shiromizu A, et al. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum. 2007;50:1370–6.
Nakadoi K, Tanaka S, Kanao H, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057–62.
Tanaka S, Haruma K, Teixeira CR, et al. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol. 1995;30:710–7.
Kobayashi H, Mochizuki H, Morita T, et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203–11.
Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94.
Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy. 2012;44:590–5.
Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol. 2014;2:292–302.
Oka S, Tanaka S, Kanao H, et al. Mid-term prognosis after endoscopic resection for submucosal colorectal carcinoma: summary of a multicenter questionnaire survey conducted by the colorectal endoscopic resection standardization implementation working group in Japanese Society for Cancer of the Colon and Rectum. Dig Endosc. 2011;23:190–4.
Nakadoi K, Oka S, Tanaka S, et al. Condition of muscularis mucosae is a risk factor for lymph node metastasis in T1 colorectal carcinoma. Surg Endosc. 2014;28:1269–76.
Miyachi H, Kudo SE, Ichimasa K, et al. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol. 2016;31:1126–32.
Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–9.
Asayama N, Oka S, Tanaka S, et al. Long-term outcomes after treatment for T1 colorectal carcinoma. Int J Colorectal Dis. 2016;31:571–8.
Yoda Y, Ikematsu H, Matsuda T, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy. 2013;45:718–24.
Belderbos TD, van Erning FN, de Hingh IH, et al. Long-term recurrence-free survival after standard endoscopic resection versus surgical resection of submucosal invasive colorectal cancer: a population-based study. Clin Gastroenterol Hepatol. 2016;. doi:10.1016/j.cgh.2016.08.041.
Japanese Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Revision 4th ed. Tokyo: Kanehara & Co., Ltd.; 1986.
Japanese Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Revision 5th ed. Tokyo: Kanehara & Co., Ltd.; 1994.
Japanese Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. 1998 6th ed. Tokyo: Kanehara & Co., Ltd.; 1998.
Japanese Society for Cancer of the Colon and Rectum. JSCCR guidelines 2005 for the treatment of colorectal cancer. Tokyo: Kanehara & Co., Ltd; 2005.
Whitlow C, Gathright JB Jr, Hebert SJ, et al. Long-term survival after treatment of malignant colonic polyps. Dis Colon Rectum. 1997;40:929–34.
Read TE, Mutch MG, Chang BW, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33–40.
Barillari P, Ramacciato G, Manetti G, et al. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Dis Colon Rectum. 1996;39:388–93.
Choi DH, Sohn DK, Chang HJ, et al. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009;52:438–45.
Rickert A, Aliyev R, Belle S, et al. Oncologic colorectal resection after endoscopic treatment of malignant polyps: does endoscopy have an adverse effect on oncologic and surgical outcomes? Gastrointest Endosc. 2014;79:951–60.
Overwater A, Kessels K, Elias SG, et al. Endoscopic resection of high-risk T1 colorectal carcinoma prior to surgical resection has no adverse effect on long-term outcomes. Gut. 2016;. doi:10.1136/gutjnl-2015-310961.
Fleischer D, Goldberg S, Browning T, et al. Detection and surveillance of colorectal cancer. JAMA. 1989;261:580–5.
Kaneko I, Tanaka S, Oka S, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;13:3829–35.
Acknowledgements
This study was conducted with a Grant-in-Aid from the Japan Agency for Medical Research and Development, AMED (15ck0106102h0102).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Tamaru, Y., Oka, S., Tanaka, S. et al. Long-term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol 52, 1169–1179 (2017). https://doi.org/10.1007/s00535-017-1318-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1318-1