Abstract
Background
Recent studies have shown important roles for activation-induced cytidine deaminase (AID), an intrinsic genome mutator, in H. pylori-associated gastric cancer development. Here, we evaluated the relationship between H. pylori-induced gastritis and AID expression from human biopsy specimens.
Methods
In 109 patients with dyspeptic symptoms who had undergone endoscopy and received biopsy of the antrum, angulus, and corpus, H. pylori infection was diagnosed by serologic test, 13C urea breath test, and histological examination. Histological scores of H. pylori, neutrophils, mononuclear cells, atrophy, and intestinal metaplasia (IM) were assessed using the updated Sydney system (USS). Immunohistochemical AID expression of the biopsy specimens was scored.
Results
Sixty of 109 (55.0 %) patients were positive for H. pylori and eradication was successful in 48 patients. AID expression in H. pylori-infected mucosa was significantly higher (p < 0.01) than in non-infected mucosa. AID expression was highest in the antrum and was significantly (p < 0.01) reduced toward the proximal portion of the stomach. For USS, multivariate analysis using linear regression revealed that mononuclear cell infiltration (p < 0.01) and IM (p < 0.05) correlated independently with AID expression. After eradication of H. pylori, AID expression was significantly decreased (p < 0.01), but was still higher than that in H. pylori-negative patients in all sites of the stomach.
Conclusions
AID expression is elevated in H. pylori-positive patients and is reduced following H. pylori eradication. Moreover, AID expression is highest in the antrum and correlated with severity of chronic inflammation and IM, suggesting an important role for AID in gastric cancer development through gastritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the discovery of Helicobacter pylori by Warren and Marshal in 1982, it has been well established that H. pylori infection plays a key role in the development of gastric cancer [1–3]. Development of gastric cancer by H. pylori infection involves a multi-step process of gastritis, which starts with active inflammation and neutrophilic infiltration and progresses to chronic inflammation with mononuclear cell infiltration and subsequent glandular atrophy, intestinal metaplasia (IM), and dysplasia, eventually leading to cancer [4]].
The updated Sydney system (USS) has been widely used for the histological evaluation of H. pylori-associated gastritis [5–8], and severity on the scale has been shown to be useful for predicting gastric cancer development [3, 5–8]. However, the molecular mechanisms underlying gastric cancer development through progression of gastritis have not been fully studied.
Various mechanisms have been considered for gastric cancer development following H. pylori infection. Among them, an important characteristic feature of cancer development is the stepwise accumulation of genetic alterations such as gene mutations and gene deletions [9, 10]. It has been shown that H. pylori can induce gene mutation in the gastric mucosa. Indeed, the mucosa in H. pylori-infected gastritis shows a considerable level of gene mutations [9–12]. We recently reported that activation-induced cytidine deaminase (AID) plays a critical role as an intrinsic genome mutator in H. pylori-induced gastric cancer development [13, 14]. In vivo, AID is exclusively expressed in B cells and contributes to diversification of antibody production by inducing both somatic hypermutation and class-switch recombination of immunoglobulin genes [11, 13–15]. Interestingly, we found that AID is expressed aberrantly in the gastric mucosa in response to H. pylori infection through NFκB activation and that it enhances genetic aberrations such as gene mutations and deletions [13, 14]. Moreover, we showed that H. pylori induces AID expression, not only directly but also indirectly, through enhancing gastric inflammation, suggesting that AID expression may reflect the risk for gastric cancer development in the gastric mucosa [13]. However, the histological relationship between AID expression and H. pylori-associated gastritis has not been well characterized.
In the present study, to elucidate the relationship between H. pylori-induced gastritis and AID expression more precisely, we assessed histological findings of USS and AID expression using a large number of biopsy specimens from the gastric mucosa of dyspeptic patients. Furthermore, we also examined the changes of AID expression in the gastric mucosa after the eradication of H. pylori.
Methods
Patients
We recruited 253 consecutive dyspeptic patients who agreed to receive upper gastrointestinal endoscopy and biopsy at three sites of the stomach to assess H. pylori-associated gastritis between 2002 and 2008 at the National Center for Global Health and Medicine (NCGM), Japan.
We excluded the following 144 patients from analysis: 3 who had a severe underlying disease; 12 who had taken non-steroidal anti-inflammatory drugs (NSAIDs); 21 who had taken anti-thrombotic drugs; 56 who had taken a proton pump inhibitor (PPI); 12 who had taken an H2-blocker; 56 who had undergone eradication of H. pylori; and 6 whose histological features could not be evaluated because of insufficient biopsy samples. More than one exclusion criterion was applied to some patients. As a result, the remaining 109 patients were included for analysis. The study protocol was approved by the ethics committee of the National Center for Global Health and Medicine, Tokyo, Japan.
Assessment of H. pylori status and eradication
H. pylori infection status was evaluated by the presence of immunoglobulin G antibody against H. pylori in the serum (HM-CAP, Enteric Products, Westbury, NY), 13C urea breath test (UBT; with a cut-off value of 2.5 ‰; Ubit, Otsuka Pharmaceuticals, Tokyo, Japan), and histological examination with toluidine blue staining. When all three methods yielded negative results, H. pylori infection was considered negative.
H. pylori-positive patients were treated with a 7-day regimen consisting of amoxicillin 750 mg, clarithromycin 200 mg (or 400 mg), and a omeprazole 20 mg or lansoprazole 30 mg or rabeprazole 10 mg twice daily. If eradication was not successful, a second regimen consisting of amoxicillin 750 mg, metronidazole 250 mg, and a omeprazole 20 mg or lansoprazole 30 mg or rabeprazole 10 mg was administered.
Eradication was confirmed by negative histological examination of gastric biopsy specimens, together with a negative 13C-UBT at least 2 months after the completion of eradication therapy. When all of the tests were negative, a patient was defined as having undergone successful eradication of H. pylori infection. After confirmation of successful eradication for a mean (SD) period of 16.6 (8.8) months, endoscopic biopsy of the same locations (antrum, angulus, and corpus) as those at the initial diagnosis was repeated.
Endoscopic assessment
All endoscopies were performed by well-trained endoscopists using a Q240 or Q260H video endoscope (Olympus, Tokyo, Japan). Endoscopic diagnosis included active duodenal ulcer (DU), active gastric ulcer (GU), and hyperplastic polyp.
Histological assessment
Biopsy specimens were taken from the following three sites of the gastric mucosa: the greater curvature of the antrum (antrum), the incisura angulus (angulus), and the greater curvature of the upper body (corpus). Thus, a total of 327 biopsy specimens were taken from 109 patients, fixed in formalin, and stained with hematoxylin and eosin and toluidine blue.
According to the USS, a score of 0–3 (0, absent; 1, mild; 2, moderate; 3, marked) was assigned to each of the following parameters: H. pylori, neutrophil infiltration, mononuclear cell infiltration, glandular atrophy, and IM [5].
Inter-observer agreement of histological findings of USS
Histological features were diagnosed by an expert pathologist (Y.L.) who was blinded to clinical information and endoscopic findings. To validate inter-reliability and the consensus of the ratings given by judges, another expert pathologist (T.I.) who was also blinded to clinical information and endoscopic findings evaluated each pathological finding and its associated USS score in 74 patients.
Assessment of AID expression
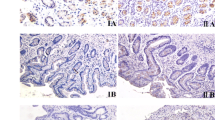
AID immunostaining was performed using a specific antibody for AID [13] and was semiquantitatively evaluated for the percentage and intensity of the positively stained gastric epithelial cells because there was no established standard (Fig. 1). We chose a field with best quality, and evaluated AID levels. The percentage score was evaluated as follows: 0 = no staining, 1 = ≤5 % stained cells, 2 = >5–10 %; 3 = >10–30 %; 4 = >30 % stained cells. An intensity score of 0, 1, 2, or 3 corresponded to no, weak, moderate, or strong staining of cells, respectively. We used some plasma cells with strong staining of AID as internal positive controls. We scored 3 when the cytoplasm was stained at the same level or more than that of plasma cells. Accordingly, immunohistochemical AID expression in each biopsy specimen was scored from 0 to 7 by combining percentage score and intensity score. Immunohistochemical analysis was evaluated by a single observer (Y.L.).
Immunohistochemical analysis of AID expression. Representative images of HE staining (a–c) and AID immunostaining (d–f) are shown. a, d Normal fundic mucosa of a H. pylori-negative patient with no AID immunostaining. b, e Fundic mucosa with intestinal metaplasia in a H. pylori-positive patient with strong AID expression (> 30 % cells and strong intensity). Arrows in e indicate epithelial cells with strong AID expression. c, f Some plasma cells were used as internal positive control (arrows)
Statistical analysis
We examined differences in patient characteristics (age, sex, and endoscopic diagnosis) and histological scores between H. pylori-positive and -negative patients. Wilcoxon’s rank sum test was used to compare age with histological score. The Chi squared test was used to compare sex ratio with endoscopic diagnosis between the two groups.
AID expression was compared between groups by Wilcoxon’s rank sum test for H. pylori infection (positive or negative), age (≥ or < 60 years), and sex (male or female) in all patients. In patients with H. pylori infection, simple linear regression analysis was used to determine the USS inflammatory parameters correlating with AID expression. Then we used a multiple linear regression model to identify the independent factors correlating with AID expression. Wilcoxon’s matched-pairs signed-rank test was used to compare AID expression with intragastric localization (antrum, angulus, and corpus).
To identify changes in AID expression before and after H. pylori eradication, we used Wilcoxon’s matched-pairs signed-rank test. We also examined the difference in AID expression between H. pylori-eradicated patients and H. pylori-negative patients using Wilcoxon’s rank sum test.
We calculated the value of agreement in pathological findings for the USS score. Inter-observer agreement was measured with kappa statistics [17, 18]. Kappa values > 0.80 denoted excellent, between 0.60 and 0.80 good, between 0.40 and 0.60 moderate, between 0.20 and 0.40 fair, and < 0.20 poor [17].
Values of p < 0.05 were considered significant. All statistical analysis was performed using Stata version 10 software (StataCorp, Lakeway Drive College Station, TX, USA).
Results
Patient characteristics
Patient baseline characteristics are shown in Table 1. Of the 109 patients, 60 (55.0 %) were positive for H. pylori. Age and sex did not differ between H. pylori-positive and -negative patients. H. pylori-positive patients comprised 10 with DU, 4 with GU, and 5 with hyperplastic polyps.
All USS scores of H. pylori density, neutrophil infiltration, monocyte infiltration, glandular atrophy, and IM at any portion of the gastric mucosa (antrum, angulus, and corpus) in H. pylori-positive patients were significantly higher than those in H. pylori-negative patients, except for glandular atrophy and IM in the corpus.
AID expression according to H. pylori infection and intragastric localization
AID expression was significantly (p < 0.01) higher in H. pylori-positive patients than in H. pylori-negative patients in the corpus, angulus, and antrum (Fig. 2a).
AID expression in different portions of the gastric mucosa. a Data from H. pylori-negative patients (white bars) and H. pylori-positive patients (black bars) are shown. †AID expression in H. pylori-positive patients was significantly higher than in H. pylori-negative patients at all sites (p < 0.01). *Significantly higher than in the corpus (p < 0.01), and **significantly higher than in the angulus (p < 0.01) in H. pylori-positive patients. ***Significantly higher than in the corpus and angulus of H. pylori-negative patients (p < 0.01). b Data of age < 60 years old (white bars) and age ≥60 years old (gray bars) are shown. No significant differences in AID expression between age groups at any sites were noted (p > 0.05). c Data from females (white bars) and males (gray bars) are shown. No significant differences in AID expression between age groups at any sites (p > 0.05) were noted
In H. pylori-positive patients, AID expression was highest in the antrum (p < 0.01), and expression significantly (p < 0.01) decreased toward the proximal part of the stomach (Fig. 2a). In H. pylori-negative patients, AID expression in the antrum was significantly higher than that of the angulus (p < 0.01) and corpus (p < 0.01) (Fig. 2a). There were no significant differences in AID expression for age group or sex at any sites of the stomach (Fig. 2b, c).
AID expression and USS scores in H. pylori-positive patients
The correlation between AID expression and USS scores in H. pylori-positive patients is shown in Table 2. AID expression correlated significantly with neutrophil infiltration (p = 0.01), mononuclear cell infiltration (p < 0.01), glandular atrophy (p < 0.01), and IM (p < 0.01) by simple linear regression analysis. However, AID expression did not correlate significantly with H. pylori density. Multivariate analysis using linear regression revealed that mononuclear cell infiltration (p < 0.01) and IM (p < 0.05) were independently correlated with AID expression (Table 2). In agreement with these data, we also found that AID expression is significantly higher in gastritis with IM than that without IM (mean AID score, gastritis with IM 5.5 versus without IM 3.6, p < 0.01).
Changes in AID expression before and after H. pylori eradication
Of the 60 H. pylori-positive patients, 50 agreed to undergo eradication therapy, and successful eradication was confirmed in 48 of these patients. USS scores of H. pylori density, neutrophil infiltration, and mononuclear cell infiltration were significantly decreased in all portions of the gastric mucosa (p < 0.01), while atrophy and IM scores were not significantly decreased after eradication (Table 3).
AID expression in H. pylori-infected patients (n = 48) was significantly (p < 0.01) decreased following eradication of H. pylori in all sites of the stomach, but expression in eradicated patients (n = 48) was still higher than that in H. pylori-uninfected patients (n = 49) in all sites of the stomach, with a significant difference in the angulus (p = 0.01) (Fig. 3).
Changes in AID expression in the gastric mucosa following H. pylori eradication. †AID expression was significantly decreased by H. pylori eradication (p < 0.01; Wilcoxon’s matched paired signed-rank test) in all three sites of the stomach. However, it was still higher than in H. pylori-negative patients in all sites of the stomach, ††especially in the angulus (p = 0.01; Wilcoxon’s rank sum test)
Inter-observer agreement of USS scores
Kappa values of H. pylori scores were moderate in the antrum and angulus (0.50 and 0.56, respectively) and good (0.61) in the corpus. The kappa value of neutrophil infiltration scores was good in the antrum, angulus, and corpus (0.60, 0.70, and 0.74, respectively). The kappa value of mononuclear cell infiltration scores was good in the antrum, angulus, and corpus (0.63, 0.68, and 0.65, respectively). The kappa value of glandular atrophy scores was fair (0.35) in the antrum and moderate in the angulus and corpus (0.43 and 0.52, respectively). The kappa value of IM scores was good in the antrum, angulus, and corpus (0.77, 0.80, and 0.80, respectively).
Discussion
Recent studies have revealed that AID plays a critical role in inflammation-associated cancer development in various digestive organs by inducing genome instability, including gene mutations and aberrations [11, 13, 15, 19]. Importantly, AID has been shown to be strongly expressed in H. pylori-positive gastritis mucosa as well as gastric cancer tissue in humans, suggesting involvement of AID in gastric cancer development [11, 13, 14]. However, the precise relationship between gastritis and AID expression has not been evaluated. In this study, we examined AID expression in the gastric mucosa of 109 patients who had undergone endoscopy with biopsy of three locations. We then confirmed that AID expression was significantly higher in H. pylori-positive patients than in H. pylori-negative patients, but it was significantly reduced by H. pylori eradication. Furthermore, USS enabled us to demonstrate that AID expression in the gastric mucosa of H. pylori-positive patients correlated significantly with gastritis severity. These data strongly suggest important roles for AID in the gastritis-to-gastric cancer sequence.
In this study, we used USS to evaluate the relationship between the degree and characteristics of gastritis and AID expression. Simple linear regression analysis showed that AID expression correlated significantly with neutrophil infiltration, mononuclear cell infiltration, glandular atrophy, and IM. Multivariate analysis revealed that only mononuclear cell infiltration and IM scores are independent factors correlating significantly with AID expression. Recently, Takeda et al. [20] demonstrated that AID expression in the non-cancerous mucosa adjacent to the tumor is associated with the severity of mononuclear cell activity, which is consistent with our study. In contrast, we could not find a significant correlation between H. pylori density and AID expression. Data regarding H. pylori density are in agreement with those of a previous study by Goto et al. [21]. However, their data, like ours, are rather unexpected, because previous studies have clearly shown direct and potent effects of H. pylori on AID expression in gastric mucosal cells [13–15]. Nevertheless, it has also been reported that not only H. pylori but also various cytokines induced during inflammation, such as tumor necrosis factor (TNF)α, interleukin (IL)1-β, and IL4, enhance AID expression [10, 11, 13, 15, 22]. Thus, the present findings that inflammatory cell infiltration, particularly mononuclear cell infiltration, correlated significantly with AID expression suggest that cytokines produced by mononuclear cells such as macrophages [23, 24] during chronic gastric inflammation may be more important stimuli than H. pylori for AID expression in the clinical setting.
The finding that IM scores correlated independently with AID expression is also somewhat surprising because inflammation is generally reduced in the gastric mucosa with severe IM [25]. Furthermore, it is believed that H. pylori colonization cannot occur in the gastric mucosa with IM [26]. Thus, the reason for the significant correlation between AID expression and IM scores is unknown. However, it is well established that IM reflects persistence of chronic gastritis [4, 25]. Therefore, it can be speculated that when chronic gastritis continues in response to long-term H. pylori infection, gastric epithelial cells switch on autonomous AID expression. The present data which showed that AID expression was still present in the gastric mucosa after H. pylori eradication at higher levels than in non-infected patients might support such a possibility. In agreement with this notion, we have often found autonomous high expression of AID in human gastric cancer cell lines [13, 21, 27].
The present study clearly showed that the expression of AID in H. pylori-positive patients was most prominent in the antrum and exhibited a linear reduction toward the proximal portion of the stomach, angulus, and corpus. H. pylori first colonizes the antral mucosa [1], and inflammation subsequently spreads to the proximal stomach [3, 28]. The mean age of our H. pylori-positive patients was 56, thus most of our patients appear to have had long-term H. pylori infection and chronic gastritis. In Japan, corpus predominant gastritis is the most prevalent form of H. pylori-induced chronic gastritis [29]. In this sense, the higher expression of AID in the antrum than in the corpus in our patients is intriguing. The reason for this phenomenon is not clear at present. H. pylori infection induces NFκB activation, and NFkB has been reported to play a central role in H. pylori-induced gastric inflammation [11, 13, 15]. Notably, NFkB has been shown to be strongly activated in the antrum [22], which might result in high AID expression in the antrum. Alternatively, the finding that AID expression in the antrum is higher than in other parts of the stomach, even in H. pylori-negative patients, may indicate that the antrum is the site where AID expression is easily induced. In this regard, it might be possible that different cellular composition between the corpus and the antrum; ex. presence or absence of parietal cells and chief cells resulted in different AID expression levels.
It has been suggested that a high concentration of bile acids plays a role in the progression of premalignant conditions in H. pylori-negative cases [30]. In addition, our previous study revealed that bile acid is a potent enhancer of AID expression [31], which might also explain, at least in part, the strong AID expression in the antrum. In any case, our data clearly showed that strong AID expression persists in the antrum during long-term H. pylori infection, which is the possible reason for the antrum being the site where gastric cancer develops most frequently [32].
The present study clearly demonstrated that AID expression was significantly reduced by H. pylori eradication. This finding appears to be compatible with data showing that H. pylori eradication reduces the risk for gastric cancer development [16, 33]. However, recent reports have also revealed that several patients developed gastric cancer even after H. pylori eradication [16, 34]. Such a result may also be in agreement with our results which showed that although reduced, AID expression after eradication was still higher in H. pylori-positive patients than in H. pylori-negative patients. In this regard, we found that although both scores of active and chronic inflammation were reduced, scores of atrophy and IM remained unchanged after eradication. Thus, the decrease of AID expression by H. pylori eradication may have been derived mainly from the reduction of chronic inflammation, while the residual AID expression may be related to atrophy and IM. Remaining atrophy and IM in the gastric mucosa after H. pylori eradication may suggest that even eradicated patients still have the risk for gastric cancer development, and thus the subsequent endoscopic follow-up may be needed. However, since the number of the patients was rather small in our study, further study with a larger number of patients will be required to clarify precise change of AID expression by H. pylori eradication.
Here, we demonstrated that AID expression is elevated in H. pylori-positive patients and is reduced following H. pylori eradication. Moreover, AID expression correlated significantly with chronic inflammation and IM and thus appears to be involved in H. pylori-induced gastric carcinogenesis. Recent studies have shown that inflammation promotes epigenetic changes during gastric carcinogenesis [35]. In addition, it has also been reported that AID expression is associated with epigenetic alteration [36]. These data further support important roles of AID in inflammation-associated gastric carcinogenesis.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- IM:
-
Intestinal metaplasia
- USS:
-
Updated Sydney system
- AID:
-
Activation-induced cytidine deaminase
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PPI:
-
Proton pump inhibitor
- 13C-UBT:
-
13C urea breath test
- DU:
-
Duodenal ulcer
- GU:
-
Gastric ulcer
References
Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–75.
Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–131.
Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
Correa P. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomark Prev. 2003;12:238s–41s.
Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
Rugge M, Genta RM, OLGA Group. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807–8.
Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–6.
Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–8.
Costa AC, Figueiredo C, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2009;14(Suppl 1):15–20.
Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239–48.
Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550–63.
Touati E, Michel V, Thiberge JM, et al. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology. 2003;124:1408–19.
Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6.
Matsumoto Y, Marusawa H, Kinoshita K, et al. Up-regulation of activation-induced cytidine deaminase causes genetic aberrations at the CDKN2b–CDKN2a in gastric cancer. Gastroenterology. 2010;139:1984–94.
Marusawa H, Chiba T. Helicobacter pylori-induced activation-induced cytidine deaminase expression and carcinogenesis. Curr Opin Immunol. 2010;22:442–7.
Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–68.
Shimizu T, Marusawa H, Endo Y. Inflammation-mediated genomic instability: roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–6.
Takeda Y, Yashima K, Hayashi A, et al. Expression of AID, P53, and Mlh1 proteins in endoscopically resected differentiated-type early gastric cancer. World J Gastrointest Oncol. 2012;4:131–7.
Goto A, Hirahashi M, Osada M, et al. Aberrant activation-induced cytidine deaminase expression is associated with mucosal intestinalization in the early stage of gastric cancer. Virchows Arch. 2011;458:717–24.
van Den Brink GR, ten Kate FJ, Ponsioen CY, et al. Expression and activation of NF-kappa B in the antrum of the human stomach. J Immunol. 2000;164:3353–9.
Pathak SK, Basu S, Bhattacharyya A, et al. TLR4-dependent NF-kappaB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cis-, trans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J Immunol. 2006;177:7950–8.
Yamauchi K, Choi IJ, Lu H, et al. Regulation of IL-18 in Helicobacter pylori infection. J Immunol. 2008;180:1207–16.
Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9.
Genta RM, Graham DY. Intestinal metaplasia, not atrophy or achlorhydria, creates a hostile environment for Helicobacter pylori. Scand J Gastroenterol. 1993;28:924–8.
Kim CJ, Song JH, Cho YG, et al. Activation-induced cytidine deaminase expression in gastric cancer. Tumour Biol. 2007;28:333–9.
Stolte M, Eidt S. Lymphoid follicles in antral mucosa: immune response to campylobacter pylori? J Clin Pathol. 1989;42:1269–71.
Naylor GM, Gotoda T, Dixon M, et al. Why does japan have a high incidence of gastric cancer? comparison of gastritis between UK and japanese patients. Gut. 2006;55:1545–52.
Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr. 2012;50:217–21.
Morita S, Matsumoto Y, Okuyama S, et al. Bile acid-induced expression of activation-induced cytidine deaminase during the development of Barrett’s oesophageal adenocarcinoma. Carcinogenesis. 2011;32:1706–12.
Olearchyk AS. Gastric carcinoma. A critical review of 243 cases. Am J Gastroenterol. 1978;70:25–45.
Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–8.
Ito M, Takata S, Tatsugami M, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol. 2009;44:365–71.
Ushijima T, Nakajima T, Maekita T. DNA methylation as a marker for the past and future. J Gastroenterol. 2006;41:401–7.
Tatemichi M, Hata H, Nakadate T. Ectopic expression of activation-induced cytidine deaminase caused by epigenetics modification. Oncol Rep. 2011;25:153–8.
Acknowledgments
We thank Ms. Hisae Kawashiro, Clinical Research Coordinator for help with data collection. This work was supported by JSPS KAKENHI 21229009, 24229005, and 24659363, Research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) from Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Health and Labour Sciences Research Grants for Research of Japan, and a grant from the National Center for Global Health and Medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagata, N., Akiyama, J., Marusawa, H. et al. Enhanced expression of activation-induced cytidine deaminase in human gastric mucosa infected by Helicobacter pylori and its decrease following eradication. J Gastroenterol 49, 427–435 (2014). https://doi.org/10.1007/s00535-013-0808-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0808-z