Abstract
Background
Wilms’ tumor 1-associating protein (WTAP) is a nuclear protein that has been associated with the regulation of proliferation and apoptosis. Although its dynamic expression and physiological functions in vascular cells have been reported, its expression and roles in cholangiocarcinoma cells are poorly characterized.
Methods
To examine the expression of WTAP in patient tissues, we performed immunohistochemistry. To examine motility of cholangiocarcinoma cells, we employed Boyden chamber, wound healing and Matrigel invasion assays, and a liver xenograft model.
Results
Immunohistochemistry in patient tissues showed WTAP overexpression in cholangiocarcinoma tissues and correlation of WTAP expression with metastasis of cholangiocarcinoma cells. Overexpression or knockdown of WTAP significantly increased or decreased the motility of cholangiocarcinoma cells. Moreover, WTAP overexpression or knockdown significantly increased or decreased tumorigenicity of cholangiocarcinoma cells in an orthotopic xenograft model. Furthermore, microarray study showed that WTAP induce the expressions of MMP7, MMP28, cathepsin H and Muc1.
Conclusion
WTAP is overexpressed in cholangiocarcinoma and regulates motility of cholangiocarcinoma cells
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma is the second most common subtype of primary hepatobiliary cancer [1–4]. The mortality rate of intrahepatic cholangiocarcinoma has increased in Japan, Western Europe, and Australia between 1979 and 1998. This higher incidence in East Asia is likely due to regional risk factors, such as heptolithiasis and liver fluke infection. An important prognostic factor for cholangiocarcinoma is metastasis, which precludes curative surgical resection. Prognosis is dependent on the presence of free margins in resected tissues and the absence of lymph nodes metastasis [3]. Increased cell invasion and migration is a key phenotypic advantage of malignant cells favoring metastasis.
Mammalian Wilms’ tumor 1-associating protein (WTAP) was first identified as a nuclear protein that associates with the Wilms’ tumor1 suppressor gene product WT1 by Little et al. [5]. The importance of WTAP is suggested by knock-out experiments; for example, WTAP-null and heterozygous mice died at E6.5 and E10.5, respectively [6, 7]. Furthermore, WTAP has been suggested to participate in cell proliferation and apoptosis in studies on vascular cells. In particular, in vascular smooth muscle cells, WTAP negatively regulated proliferation, and knockdown of its expression by RNA interference increased DNA synthesis and proliferation [8, 9], whereas its overexpression had opposite effects. The role of WTAP in the apoptosis of vascular smooth muscle cells was described by Small et al. [10], who found that insulin-like growth factor-1 (IGF-1) confers antiapoptotic properties on smooth muscle cells by regulating WTAP abundance. However, with the exceptions of cell proliferation and apoptosis, its roles in other cellular functions have been poorly characterized.
Three molecular mechanisms have been suggested to explain how WTAP contributes to cellular processes. The first is that WTAP regulates binding of WT1 to its transcriptional target DNA. In vascular smooth muscle cells, WTAP suppressed the expression of amphiregulin, a direct target of WT1 and a sensitive indicator of WT1 transcriptional activity [8, 11]. Another target gene, Bcl-2 was also regulated by WTAP [8, 12]. The second is that WTAP regulates RNA splicing. Localization of WTAP to interchromatin granule clusters, where RNA splicing factors are assembled, modified, or stored, [5, 13] and the identification of WTAP as a component of spliceosome [14] support this possibility. Furthermore, immunodepletion of WTAP from HeLa cell extracts was shown to alter the splicing of exogenous transformer pre-mRNA [15]. The third proposed mechanism relates the stabilization of mRNA by WTAP; for example, in human umbilical vein endothelial cells, WTAP has been reported to regulate cell proliferation by stabilizing cyclin A2 mRNA [6].
Despite its ubiquitous distribution, dynamic changes in WTAP expression have been described. Its expression was found to be substantially up-regulated when vascular smooth muscle cells shift from a synthetic, proliferative state to a nonproliferative, contractile state, [8]. Furthermore, during the early period after rat carotid artery injury, the expression of WTAP is low, but later when smooth muscle cells stop proliferating its expression is high [8]. However, the regulation of WTAP expression in other tissues has been poorly characterized.
In the present study, we examined the expressional status and roles of WTAP in cholangiocarcinoma cells.
Materials and methods
Tissue specimens
Tissue samples from 27 unrelated Korean patients with intrahepatic cholangiocarcinoma who were treated at Pusan National University Hospital were obtained at the time of surgical resection between 2009 and 2012. Tumor tissues were obtained after the patients’ informed consent was obtained under a protocol reviewed and approved by the institutional review board of Pusan National University Hospital. During the surgical resection, dissection of regional lymph nodes was performed in all cases. The tissues were fixed in 10 % buffered formaldehyde solution for pathologic diagnosis and immunohistochemical staining. Histopathologic diagnosis of each neoplastic tissue was performed by the Department of Pathology, Pusan National University Hospital. Clinicopathologic staging was determined by the TNM classification. All patients had cholangiocarcinoma that was confirmed histologically, and tumor samples were checked to ensure that tumor tissue was present in more than 80 % of the specimen’s area.
Cell culture and transfection
HuCCT1 and SNU1196 cholangiocarcinoma cells were used for this study. HuCCT1 cell line was purchased from the Health Science Research Resources Bank (Osaka, Japan). SUN1196 cells lines were purchased from the Korean Cell Line Bank (Seoul, Korea). HuCCT1 and SUN1196 cells were cultured in RPMI1640 supplemented with 10 % FBS and 1× penicillin/streptomycin (1 × P/S). WTAP siRNA (Bioneer, Daejeon, Korea) and scrambled (SCR) siRNA (Dhamacon, Lafayette, CO, USA) were purchased. Cells were transfected with WTAP, or SCR siRNA using Dhamafect reagent (Dhamacon, Lafayette, CO, USA) according to the manufacturer’s instruction. SCR siRNA was used as negative control. The sequences of WTAP siRNA duplex were as follows: 5′-CAG AUC UUA ACU CUA AUG A-3′, 5′-CCU UGU AAU GCG ACU AGC A-3′, and 5′-GAG AUG CAA GAG UGU ACU A-3′.
Overexpression of WTAP
The DNA expression-construct pCMV6-XL5 + WTAP (Thermo Scientific Open Biosystems, Huntsville, AL, USA) was used to drive WTAP overexpression and G418 was used for selection. pCMV6-XL5 empty vector was used as a control vector. Cells were transfected with pCMV6-XL5 + WTAP/pEGFPN1 at a ratio 5:2 or pCMV6-XL5 using FuGENE HD (Roche, Nutley, NJ, USA), in accordance with the manufacturer’s instructions. Two days after transfection, the cells were transfer-selection-cultured for 2 weeks under the pressure of G418 (300 μl/ml, Sigma-Aldrich, St. Louis, Mo, USA) in culture medium.
Real-time PCR
Total RNA from cells were extracted using RNeasy Mini kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s protocol. cDNA was synthesized with MMLV reverse transcriptase (Promega, Madison, WI, USA), dNTP and oligo(dT) primers. Real-time RT-PCR was carried out using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster city, CA, USA) in the ABI Prism 7500 sequence detector (Applied Biosystems, Foster city, CA, USA) according to the manufacturer’s instruction. The primer sequences were as follows: WTAP v1 (F: 5′-TGT GCT GTG TAA GGG CAT TCG TAC TCA TGC-3′, R: 5′-ACT GGG CAA ACT TGG CAG TCA TAA ACC CAC-3′), MMP7 (F: 5′-TCT GGA CGG CAG CTA TGC GAC TCA C-3′, R: 5′-TCA CTC ATG CCT CCC GCC TCC TG-3′), MMP28 (F: 5′-AGT TAT GCG GCC TGG GCT GAG AGG-3′, R: 5′-AGG CCA GTT CAC CAG GCG GTA GG-3′), CTSH (F: 5′-GTG CCG CCG AAC TGT GCG TGA-3′, R: 5′- CTG CAG CCT GTG GTG GTA CTC CTC C-3′), MUC1 (F: 5′-TGT GCC CCC TAG CAG TAC CGA TCG T-3′, R: 5′-CAC TCA GCT CAG CGG GCG ACG-3′), S100A4 (F: 5′-ACA ACC CTC TCT CCT CAG CGC TTC T-3′, R: 5′-AGG TGG ACA CCA TCA CAT CCA GGG C-3′), GAS6 (F: 5′-CGA CCT CCG TGC CGT GCC TCT-3′, R: 5′-ACG TGC TCT TGG CCG TCG CAG-3′), S100P (F: 5′-GCA CCA AGA GGC TGC CAG TGG GA-3′, R: 5′-GCT GCC CTC GCT GCC CGA ATA-3′), CCL2 (F: 5′-TGG ACC ACC TGG ACA AGC AAA CCC A-3′, R: 5′-AGG GTG TCT GGG GAA AGC TAG GGG A-3′), and β-actin (F: 5′-CAA GAG ATG GCC ACG GCT GC-3′, R: 5′-TCC TTC TGC ATC CTG TCG GC-3′). β-actin was used as a loading control and all signals were normalized to β-actin.
Western blotting
Western blotting was performed as described before [16]. Following antibodies were used; anti-WTAP (HPA010550, SIGMA-Aldrich, St Louis, MO, USA, 1:5,000), β-actin antibody (Abcam, Cambridge, MA, USA, 1:10,000) and HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA, 1:5,000).
Immunohistochemistry
After deparaffinization and rehydration, the slides were subjected to 0.3 % hydrogen peroxide for 30 min to quench endogenous peroxidase activity. Blocking was performed with the 10 % normal donkey serum (NDS), 1 % BSA in 1× PBS. Anti-WTAP antibody (HPA010550, SIGMA-Aldrich, St Louis, MO, USA) binding was performed 1:300 dilution in blocking buffer overnight at 4 °C, secondary antibody (horseradish peroxidase-conjugated anti-rabbit, 1:200) binding for 2 h at RT, detection with HRP (Vector Laboratories) using the DAB substrate kit (Vector Laboratories). The percentage of cells stained for WTAP in a given section was graded on a scale of 1–4 (1, <24 %; 2, 25–49 %; 3, 50–74 %; 4, 75–100 %). The intensity of the tumor cell staining was determined relatively to that observed in adjacent hepatocytes (0, 1, 2, 3 for basal, weak, moderate, and strong). The overall staining was then represented by a composite score (product of the above 2 scores, ranging from 0 to 12). Statistical significance was evaluated by the Mann–Whitney U test. P < 0.05 was considered significant.
Cell proliferation assay
One day after transfection with siRNA, the medium was replaced with 1 % FBS or 10 % FBS medium. Three or five days post- transfection, 10 μl of Ez-Cytox (ITSBIO, Seoul, Korea) was added and incubated for 2 h under normal cell culture conditions. Cell viability was measured by absorbance at 450 nm using an ELISA reader (TECAN, Mannedorf, Switzerland).
Boyden chamber assay
A modified Boyden chamber (Neuro Probe, Gaithersburg, MD, USA) was used as described before [16]. The bottom chamber of the transwell chamber was filled with the medium containing 10 % FBS or epidermal growth factor (EGF, 100 ng/ml). Two days after transfection with scrambled (SCR) or WTAP siRNA, cells were seeded into the upper chamber of the transwell at a density of 5 × 104 cells/ml in 50 μl of serum-free medium. The rest of assay was performed as described before [16].
Wound healing assay
One day after transfecting WTAP siRNA into HuCCT1 or SUN1196 cells in a 6-well plate, cells were transferred to a 48-well. The rest of assay was performed as described before [16].
Matrigel invasion assay
One day after transfection with SCR or WTAP siRNA, cells were seeded at a density of 5 × 104 cells/ml in serum-free medium, into 8-μm porous BioCoat Matrigel chamber inserts (BD Biosciences, USA), and placed in wells filled with 70 μl of medium supplemented with 10 % fetal bovine serum (FBS) or EGF (100 ng/ml) as a chemoattractant. The rest of assay was performed as described before [16].
Liver xenograft assay
HuCCT1 cells were directly injected into livers of nude mice. After eight weeks, mice were sacrificed. Tissues were immediately fixed in 4 % paraformaldehyde (PFA) and paraffin blocks were prepared. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Pusan National University Institutional Animal Care and Use Committee (PNUIACUC) approved the experimental procedures.
cDNA microarray
Total RNA was extracted from HuCCT1-WTAP and HuCCT1 mock cells using RNeasy Mini kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s protocol. Quantified RNA was then used for microarray analysis on Human HT-12_v4_Bead chips (Illumina Inc., San Diego, CA, USA). Total RNA samples were labeled using the Illumina TotalPrep RNA Amplification Kit (Ambion, Applied Biosystem, CA, USA) for cDNA synthesis and in vitro transcription. Single-stranded RNA (cRNA) was generated and labeled by incorporating biotin-NTP (Ambion). A total of 0.5 μg of biotin-labeled cRNA were hybridized at 58 °C for 16 h to Illumina’s Human HT-12_v4_BeadChip (Illumina). The hybridized biotinylated cRNA was detected with streptavidin-Cy3 and quantified using a BeadArray Reader Scanner (Illumina) according to the manufacturer’s instructions. Array data were processed and analyzed by Illumina BeadStudio version 3.0 software (Illumina). Scanned data were normalized by the quantile–quantile normalization method and log-transformed (by base two). All data are MIAME compliant and the raw data were submitted to the public repository (NCBI’s GEO Accession Number: GSE 32134).
Data analysis
Results are presented as mean ± SD. Differences between the mean values of two groups were evaluated using the nonparametric Mann–Whitney U-test or the Student’s t-test (unpaired). For comparison of more than three groups, one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons was used. * indicates a P value of <0.05, which was considered statistically significant.
Results
WTAP was overexpressed in cholangiocarcinoma
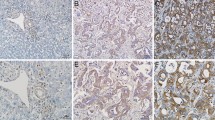
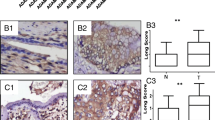
To examine the expressional status of WTAP in cholangiocarcinoma, we carried out immunohistochemistry using patient tissues (n = 27). To examine the specificity of antibody, we examined the staining pattern of WTAP in hepatocytes and epithelial cells of the small intestine. Although not all hepatocytes expressed WTAP, its staining pattern in hepatocytes was mostly cytoplasmic or membranous (Supplementary Fig. 1). However, the nuclear staining was clearly observed in epithelial cells of the small intestine. The staining pattern of WTAP in cholangiocarcinoma cells was mostly nuclear (Fig. 1). Although the expression of WTAP was noticed in the normal bile duct, WTAP protein was widely overexpressed in the cancerous areas of cholangiocarcinoma tissues as compared with non-cancerous areas (Fig. 1a). Notably, metastatic cholangiocarcinoma cells inside lymph nodes or vessels in the peritumoral region of the liver overexpressed WTAP (Fig. 1b). To correlate the expression of WTAP with the clinicopathologic characteristics of patients, we quantified its expression using a composite score which is produced by the percentage of positive tumor cells and the staining intensity. The intensity of the tumor cell staining was graded as shown in Fig. 1a. The expression level of WTAP was not correlated with age or sex of patients with cholangiocarcinoma (data not shown). However, the WTAP expression correlated with the TNM stage grading of the cholangiocarcinoma. The WTAP expression was significantly higher in the group with advanced cholangiocarcinoma than in the group with early stage cancer (Mann–Whitney U test, P < 0.05, Fig. 1c). Moreover, the WTAP expression level was significantly higher in the cholangiocarcinoma group with lymph node metastasis or vascular invasion that in the group without lymph node metastasis (Mann–Whitney U test, P < 0.05, data not shown) or vascular invasion (Mann–Whitney U test, P < 0.05, Fig. 1d).
Overexpression of WTAP in cholangiocarcinoma. Immunohistochemical staining was preformed to evaluate WTAP expression in cholangiocarcinoma. Different grades in the intensity of WTAP staining are presented (a). The overexpression of WTAP was noted in metastatic cholangiocarcinoma cells which were located within a lymph node or a vessel in the peritumoral region of the liver (b). Scale bar 50 μm. States of WTAP protein expression in patients with early stages (I and II) of cholangiocarcinoma (n = 17) and patients with advanced stages (III and IV) of cholangiocarcinoma (n = 10) are presented (c). WTAP protein expression in advanced stages is significantly higher than in the early stages (P < 0.05, Mann–Whitney U test). States of WTAP protein expression in patients with vascular invasion (n = 11) and in patients without vascular invasion (n = 16) are presented (d). WTAP expression in patients with vascular invasion is significantly higher than in patients without vascular invasion (P < 0.05, Mann–Whitney U test)
Roles of WTAP in the proliferation, migration and invasion of cholangiocarcinoma cells
To examine roles of WTAP in cholangiocarcinoma, we knocked-down or overexpressed WTAP using siRNA or cDNA, respectively. We checked the efficiency of the modulation of WTAP gene by western blotting or real-time PCR (Fig. 2a–d). To ensure that more than 80 % of cells were transfected by siRNA, we optimized the condition of siRNA transfection (data not shown). WTAP siRNA (100 nM) decreased the protein and mRNA levels of WTAP in HuCCT1 cells compared to SCR siRNA by 83.8 and 46.4 %, respectively. The expression of WTAP in SNU1196 cells was also similarly down-regulated by the siRNA (data not shown). WTAP overexpression by cDNA transfection increased the protein and mRNA levels of WTAP in HuCCT1 cells compared to the control vector by 47.6 and 114.4 %, respectively.
Role of WTAP in the proliferation of cholangiocarcinoma cells. Western blotting (a, b) and real-time PCR (c, d) were used to determine the efficiency of WTAP knockdown (a, c) or overexpression (b, d) in HuCCT1 cells. Two days after transfection with scrambled (SCR, 100 nM) or WTAP siRNA (100 nM), HuCCT1 cells were collected, and RNA and protein were purified. β-actin was used for normalization. Results are expressed as the mean ± SDs of three independent experiments (*P < 0.01, Student’s t-test). Effects of the knockdown or overexpression of WTAP on the proliferation of HuCCT1 (e) or SNU1196 (f) cholangiocarcinoma cells were presented. Cell proliferation assay was performed after transfection with SCR (100 nM) or WTAP siRNA (100 nM). Effect on proliferation was examined in the presence of 1 or 10 % FBS. Results are the mean ± SDs of three independent experiments performed in quintuplicate (*P < 0.01, Student’s t test)
Next, we examined the role of WTAP in proliferation of cholangiocarcinoma cells. Three and five days after transfection with SCR or WTAP siRNA, cell proliferation assay was carried out. Knockdown or overexpression of WTAP did not affect proliferation of HuCCT1 cholangiocarcinoma cells in the presence of 1 or 10 % FBS 3 (data not shown) or 5 days (Fig. 2e) after transfection. The proliferation of SNU1196 cells was inhibited by WTAP siRNA compared to SCR siRNA in the presence of 1 % FBS by 26.2 % 5 days (Fig. 2f) not 3 days (data not shown) after transfection. However, in the presence of 10 % FBS, the proliferation of SNU1196 was not affected by WTAP siRNA (Fig. 2f).
To determine the role of WTAP during cholangiocarcinoma cell migration, we conducted studies using a Boyden chamber assay. Because Boyden chamber assays were finished within 4–6 h after seeding with the same number of cells, WTAP siRNA or overexpression did not affect the proliferation of cancer cells (data not shown). Knockdown of WTAP inhibited migration of cholangiocarcinoma cells (Fig. 3). WTAP siRNA inhibited the FBS- and EGF-induced migrations of HuCCT1 cells compared to SCR siRNA by 48.9 and 52.0 %, respectively (Fig. 3a, b). Migration of SNU1196 cells was also similarly inhibited by WTAP siRNA (data not shown). In contrast, overexpression of WTAP increased FBS- and EGF-induced migration of HuCCT1 cells compared to the control vector by 72.1 and 62.6 %, respectively (Fig. 3a, c). Furthermore, we confirmed this effect on cancer cell migration by performing wound healing assays in HuCCT1 cells (Fig. 4). 10 % FBS or EGF (100 ng/ml) did not affect the proliferations of HuCCT1 and SNU1196 cells during wound healing assays (data not shown). These results led us to examine the role of WTAP in the invasion of cholangiocarcinoma cells. In a Matrigel invasion assay, WTAP siRNA inhibited the FBS- and EGF-induced invasion of HuCCT1 cells compared to SCR siRNA by 50.6 and 61.1 %, respectively (Fig. 5a, b). Moreover, knockdown of WTAP inhibited the FBS-induced invasion of SNU1196 cells compared to SCR siRNA by 64.0 % (Fig. 5a, c). In contrast, overexpression of WTAP increased the FBS-induced invasion of HuCCT1 cells compared to the control vector by 77.4 % (Fig. 5a, d). WTAP siRNA or overexpression did not affect the proliferation of cholangiocarcinoma cells during the invasion assays (data not shown).
WTAP regulated the migration of cholangiocarcinoma cells. To examine cancer cell migration, Boyden chamber assay was used. WTAP siRNA significantly inhibited the FBS- or EGF-induced migration compared to SCR siRNA in HuCCT1 (a, b). Overexpression of WTAP increased the migration of HuCCT1 cells (a, c). EGF (100 ng/ml) or 10 % FBS was used to induce chemotaxis. Two days after transfection with SCR (100 nM) or WTAP (100 nM) siRNA, migration assays were performed. Four or six hours later after addition of EGF or FBS in Boyden chamber assay, cells were fixed, stained with Diff–quik solution, and counted. Results are the mean ± SDs of three independent experiments performed in quintuplicate (*P < 0.01, Student’s t test)
WTAP regulated invasion of cholangiocarcinoma cells. A Matrigel invasion assay was used to measure invasion of cancer cells. WTAP siRNA significantly inhibited FBS- and EGF-induced invasion compared to SCR siRNA in HuCCT1 (a, b) or SNU1196 (a, c) cells. Overexpression of WTAP significantly increased invasion compared to the control vector (a, d). EGF (100 ng/ml) or 10 % FBS was used to induce invasion. Two days after transfection with 100 nM WTAP siRNA or 100 nM scrambled (SCR) siRNA, invasion assays were performed. Representative staining of invaded cells was presented (a). Invaded cells were counted and the data are presented as graphs (b–d). Results are the mean ± SDs of three independent experiments performed in triplicate (b–d, *P < 0.01, Student’s t-test)
WTAP enhanced tumorigenic efficacy of cholangiocarcinoma cells in a liver xenograft model
To examine an effect of WTAP on the in vivo behavior of cholangiocarcinoma cells, we injected HuCCT1 cells (5 × 105 cells) overexpressing WTAP or empty vector into the liver of nude mice (n = 6) and examined livers 8 weeks later. Interestingly, overexpression of WTAP significantly increased the size of tumors (Fig. 6). After we injected HuCCT1 cells (2 × 106 cells) transfected with WTAP siRNA or SCR siRNA, we examined livers 8 weeks later. Knockdown of WTAP significantly reduced the size of tumors (Fig. 6). In the histological examination of xenograft tissues, cholangiocarcinoma cells growing inside liver tissues were easily identified (Fig. 6).
WTAP enhanced tumorigenic efficiency in an orthotopic xenograft model. Cholangiocarcinoma cells (5 × 105 cells) overexpressing WTAP gene (WTAP-over) or control vector (Mock) were injected into liver and mice (n = 6) were examined 8 weeks later. Overexpression of WTAP significantly increased the size of tumors. Cholangiocarcinoma cells (2 × 106 cells) transfected with WTAP siRNA (siWTAP) or SCR siRNA (SCR) were injected into liver and mice (n = 6) were examined 8 weeks later. Knockdown of WTAP significantly reduced the size of tumors. The photographs show representative livers for each group and H&E stainings of xenografts. Dashed lines and asterisks indicate tumor regions and xenografted cancer cells, respectively. Rectangles indicate the magnified regions. Scale bar 100 μm
WTAP-induced changes in mRNA expression
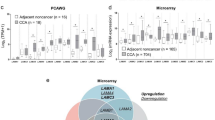
To investigate possible underlying mechanisms of WTAP-regulated motility, we performed a cDNA microarray analysis. Interestingly, many metastasis-associated genes such as MMP7, MMP28, Cathepsin H, Muc1, S100β4, and GAS6 were significantly induced by WTAP overexpression (Table 1). These changes were further confirmed by real time PCR (Fig. 7).
WTAP induced genes which regulate motility of cells. Real-time PCRs were conducted using primers specific for the indicated genes in HuCCT1 cells overexpressing WTAP or control vector. Results are the mean ± SDs of three independent experiments performed in quintuplicate (*P < 0.01, Student’s t test)
Discussion
WTAP is a nuclear protein which has mainly been described in the context of cell proliferation and apoptosis of vascular cells. However, the present study shows that WTAP is overexpressed in cholangiocarcinoma and that it regulates motility of cholangiocarcinoma cells.
The migration and invasion of cholangiocarcinoma cells greatly affect the prognosis of cholangiocarcinoma patients. The present study shows for the first time that WTAP participates in the regulation of the migration and invasion of cholangiocarcinoma cells. In the present study, Boyden chamber chemotaxis assay and Matrigel invasion assay showed that WTAP knock-down decreased but its overexpression increased the migration and invasion of cholangiocarcinoma cells. Moreover, in vivo xenograft study showed that overexpression or knockdown of WTAP increased or decreased the tumorigenicity of these cells, respectively. Finally, WTAP was found to be overexpressed in metastatic cholangiocarcinoma cells inside lymph nodes or vessels.
Then what is the molecular mechanism underlying the regulation of migration and invasion of cholangiocarcinoma cells by WTAP? In the present study, the microarray analysis showed that the expressions of MMP7, MMP28, and cathepsin H were increased by overexpression of WTAP. The increase of these enzymes, which can degrade extracellular matrix, can explain the increased invasion of cholangiocarcinoma cells by WTAP in the Matrigel invasion assay. Moreover, it can also explain the overexpression of WTAP in metastatic cholangiocarcinoma cells inside lymph nodes or vessels. In addition, WTAP overexpression was found to induce the expression of Muc1, which has been shown to regulate EGFR activity which regulates motility of cancer cells [17]. If WTAP can regulate EGFR activity through the induction of Muc1, it may explain how WTAP regulates the migration and invasion of cholangiocarcinoma cells. In the future, the question of how WTAP regulates migration and invasion of cholangiocarcinoma cells at the molecular level needs to be examined.
Various kinds of molecules which can affect migration and invasion of cholangiocarcinoma cells have been reported. Because cholangiocarcinoma can evolve from chronic inflammation, inflammation-related molecules have been shown to affect the migration and invasion. Tumor necrosis factor (TNF)-α, a proinflammatory cytokine, induced the expression of MMP9 through the cyclooxygenase (COX)-2 pathway [18]. Moreover, it also affected migration and invasion of cholangiocarcinoma cells by inducing the expression of CXCR4 [19] and Snail [20]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which was also induced by inflammation, promoted the migration and invasion through NF-κB pathway [21]. Overexpression of microsomal prostaglandin E synthase-1 (mPGES-1), which regulates the migration and invasion through the activation of EGF/phosphoinositide 3-kinase/AKT pathway, was reported in cholangiocarcinoma patients [22]. Growth factors including hepatocyte growth factor (HGF) and EGF can regulate migration and invasion of cholangiocarcinoma cells. HGF treatment activated the RAS-MEK-ERK cascade and induced cholangiocarcinoma cell invasion, which was reduced by inhibition of c-MET with siRNA or inhibition of MEK by U0126 [23]. EGF regulated the invasion and metastasis through the regulation of AKT and FOXO4 [24]. Twenty-two percent of cholangiocarcinoma patients showed K-RAS mutation, which was correlated with nodal metastasis and patients’ prognosis [25]. The amplification of ERBB2 was reported in cholangiocarcinoma patients [26]. Epithelial-mesenchymal transition (EMT) is an early step in the metastasis of cancer cells. Overexpression of transforming growth factor (TGF)-β, a critical regulator of EMT and migration of cancer cells, was observed in cholangiocarcinoma patients [27, 28]. Moreover, bile acids also regulated EMT of cholangiocarcinoma through the induction of Snail [29]. Interestingly a recent paper showed miR-200c can regulate migration and invasion of cholangiocarcinoma cells through regulation of EMT [30]. In that study, activation or inactivation of miR-200c resulted in reduction or induction of EMT. Abnormalities of the WNT signaling pathway, which can regulate EMT of cancer cells, have been observed in cholangiocarcinoma patients [31–33]. Aberrant action of WNT signaling which was indicated by nuclear localization of β-catenin was reported in 15 % of cholangiocarcinoma patients [32], and hypermethylation of adenomatous polyposis coli (APC) gene was reported in about 27 % of cholangiocarcinoma patients [33]. Other numerous molecules including NUPR1 [34], CDH3 [35], CD24 [36], CD44 [37], LGALS3 [38], MARCKS [39], NGAL [40], L1 [41], FAPB5 [42], GABA [43], RECK [44], miR-124 [45], ECM1 [46], have been associated migration or invasion of cholangiocarcinoma cells.
The present study does not support the notion that WTAP plays a critical role in the proliferation of cholangiocarcinoma cells. In the presence of 1 % FBS, knock-down of WTAP slightly reduced the proliferations of SNU1196 cells. However, in the presence of 10 % FBS, no significant decrease in proliferation was observed. Moreover, overexpression of WTAP did not affect the proliferation of HuCCT1 cells. The previous studies suggested the association of WTAP with cell proliferation based on its expression pattern [8]. However, its role in cell proliferation is controversial, depending on cell type. In vascular smooth muscle cells, WTAP negatively regulates proliferation, and knockdown of its expression by RNA interference increased DNA synthesis and proliferation [8], whereas its overexpression had the opposite effects. However, in endothelial cells, overexpression of WTAP did not affect cell proliferation and its knockdown decreased proliferation [6]. These results suggest that the role of WTAP in proliferation might be cell-type specific.
In the present study, overexpression of WTAP in cholangiocarcinoma tissues was found for the first time. Although a previous study showed that WTAP is expressed in adult tissues including brain, thymus, heart, lung, liver, spleen and skeletal muscle [5], dynamic change in its expression has been reported in vascular smooth muscle cells [8]. In the present study, we observed overexpression of WTAP in the cancerous area of patients’ tissues although the basal expression of WTAP was noted in the non-cancerous area. Moreover, the expression level of WTAP was well correlated with metastasis of cholangiocarcinoma cells into lymph node and vessels, and the clinical stage grades of patients with cholangiocarcinoma. Because the number of patients’ tissues examined in this study is not enough for generalization, more thorough study is needed in the future.
References
Kato I, Kuroishi T, Tominaga S. Descriptive epidemiology of subsites of cancers of the liver, biliary tract and pancreas in Japan. Jpn J Clin Oncol. 1990;20:232–7.
Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet. 1997;350:1142–3.
Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–79.
Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–7.
Little NA, Hastie ND, Davies RC. Identification of WTAP, a novel Wilms’ tumour 1-associating protein. Hum Mol Genet. 2000;9:2231–9.
Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, et al. Wilms’ tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc Natl Acad Sci USA. 2006;103:17278–83.
Naruse C, Fukusumi Y, Kakiuchi D, Asano M. A novel gene trapping for identifying genes expressed under the control of specific transcription factors. Biochem Biophys Res Commun. 2007;361:109–15.
Small TW, Bolender Z, Bueno C, O’Neil C, Nong Z, Rushlow W, et al. Wilms’ tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells. Circ Res. 2006;99:1338–46.
Small TW, Penalva LO, Pickering JG. Vascular biology and the sex of flies: regulation of vascular smooth muscle cell proliferation by wilms’ tumor 1-associating protein. Trends Cardiovasc Med. 2007;17:230–4.
Small TW, Pickering JG. Nuclear degradation of Wilms tumor 1-associating protein and survivin splice variant switching underlie IGF-1-mediated survival. J Biol Chem. 2009;284:24684–95.
Lee SB, Huang K, Palmer R, Truong VB, Herzlinger D, Kolquist KA, et al. The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell. 1999;98:663–73.
Mayo MW, Wang CY, Drouin SS, Madrid LV, Marshall AF, Reed JC, et al. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 1999;18:3990–4003.
Saitoh N, Spahr CS, Patterson SD, Bubulya P, Neuwald AF, Spector DL. Proteomic analysis of interchromatin granule clusters. Mol Biol Cell. 2004;15:3876–90.
Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–5.
Ortega A, Niksic M, Bachi A, Wilm M, Sanchez L, Hastie N, et al. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNA splicing. J Biol Chem. 2003;278:3040–7.
Jeon TY, Han ME, Lee YW, Lee YS, Kim GH, Song GA, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–8.
Hisatsune A, Nakayama H, Kawasaki M, Horie I, Miyata T, Isohama Y, et al. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun. 2011;405:377–81.
Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, et al. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829–41.
Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, et al. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–68.
Zhang K, Zhaos J, Liu X, Yan B, Chen D, Gao Y, et al. Activation of NF-B upregulates Snail and consequent repression of E-cadherin in cholangiocarcinoma cell invasion. Hepatogastroenterology. 2011;58:1–7.
Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–36.
Lu D, Han C, Wu T. Microsomal prostaglandin E synthase-1 inhibits PTEN and promotes experimental cholangiocarcinogenesis and tumor progression. Gastroenterology. 2011;140:2084–94.
Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, et al. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 2006;136:78–84.
Lee MJ, Yu GR, Yoo HJ, Kim JH, Yoon BI, Choi YK, et al. ANXA8 down-regulation by EGF-FOXO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology. 2009;137:1138–50, 50 e1–9.
Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy. Ann Surg Oncol. 2012;19(Suppl 3):S675–81.
Ukita Y, Kato M, Terada T. Gene amplification and mRNA and protein overexpression of c-erbB-2 (HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by fluorescence in situ hybridization, in situ hybridization, and immunohistochemistry. J Hepatol. 2002;36:780–5.
Benckert C, Jonas S, Cramer T, Von Marschall Z, Schafer G, Peters M, et al. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63:1083–92.
Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, et al. E/N-cadherin switch mediates cancer progression via TGF-beta-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105:1885–93.
Fukase K, Ohtsuka H, Onogawa T, Oshio H, Ii T, Mutoh M, et al. Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci. 2008;99:1785–92.
Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792–803.
Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ, et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer.
Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, et al. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–5.
Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161:1015–22.
Kim KS, Jin DI, Yoon S, Baek SY, Kim BS, Oh SO. Expression and roles of NUPR1 in cholangiocarcinoma cells. Anat Cell Biol. 2012;45:17–25.
Baek S, Lee YW, Yoon S, Baek SY, Kim BS, Oh SO. CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells. Anat Cell Biol. 2010;43:110–7.
Keeratichamroen S, Leelawat K, Thongtawee T, Narong S, Aegem U, Tujinda S, et al. Expression of CD24 in cholangiocarcinoma cells is associated with disease progression and reduced patient survival. Int J Oncol. 2011;39:873–81.
Pongcharoen P, Jinawath A, Tohtong R. Silencing of CD44 by siRNA suppressed invasion, migration and adhesion to matrix, but not secretion of MMPs, of cholangiocarcinoma cells. Clin Exp Metastasis. 2011;28:827–39.
Junking M, Wongkham C, Sripa B, Sawanyawisuth K, Araki N, Wongkham S. Decreased expression of galectin-3 is associated with metastatic potential of liver fluke-associated cholangiocarcinoma. Eur J Cancer. 2008;44:619–26.
Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A, et al. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 2010;101:658–65.
Nuntagowat C, Leelawat K, Tohtong R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin Exp Metastasis. 2010;27:295–305.
Min JK, Kim JM, Li S, Lee JW, Yoon H, Ryu CJ, et al. L1 cell adhesion molecule is a novel therapeutic target in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2010;16:3571–80.
Jeong CY, Hah YS, Cho BI, Lee SM, Joo YT, Jung EJ, et al. Fatty acid-binding protein 5 promotes cell proliferation and invasion in human intrahepatic cholangiocarcinoma. Oncol Rep. 2012;28:1283–92.
Huang Q, Liu C, Wang C, Hu Y, Qiu L, Xu P. Neurotransmitter gamma-aminobutyric acid-mediated inhibition of the invasive ability of cholangiocarcinoma cells. Oncol Lett. 2011;2:519–23.
Namwat N, Puetkasichonpasutha J, Loilome W, Yongvanit P, Techasen A, Puapairoj A, et al. Downregulation of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is associated with enhanced expression of matrix metalloproteinases and cholangiocarcinoma metastases. J Gastroenterol. 2011;46:664–75.
Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou Q, et al. Epigenetic regulation of miR-124 by Hepatitis C Virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2011;586:3271–8.
Xiong GP, Zhang JX, Gu SP, Wu YB, Liu JF. Overexpression of ECM1 contributes to migration and invasion in cholangiocarcinoma cell. Neoplasma. 2012;59:409–15.
Acknowledgments
This work was supported by Medical Research Institute Grant (2009-22), Pusan National University Hospital, the medical research centre program of Ministry of Education, Science and Technology/Korea Science and Engineering Foundation (2011-0006190), a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The biospecimens for this study were provided by the Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.-J. Jo and H.-E. Shim equally contributed to this work.
Electronic supplementary material
535_2013_748_MOESM1_ESM.pdf
Supplementary Fig. 1. Staining patterns of WTAP in hepatocytes and epithelial cells of the small intestine. Although not all hepatocytes expressed WTAP, its staining pattern in hepatocytes was mostly cytoplasmic or membranous. However the nuclear staining was clearly observed in epithelial cells of the small intestine. Scale bar, 50 μm (PDF 212 kb)
Rights and permissions
About this article
Cite this article
Jo, HJ., Shim, HE., Han, ME. et al. WTAP regulates migration and invasion of cholangiocarcinoma cells. J Gastroenterol 48, 1271–1282 (2013). https://doi.org/10.1007/s00535-013-0748-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0748-7