Abstract
Background
Surgery is critical in the management of gastro-oesophageal cancer, and the addition of neo-adjuvant chemotherapy has proved to be of benefit. The calpain system has been implicated in tumour progression and response to various anti-cancer therapies, and therefore expression of the system was determined in this tumour type.
Methods
Two cohorts of gastro-oesophageal adenocarcinomas were investigated for calpain-1, calpain-2, calpain-9 and calpastatin expression using conventional immunohistochemistry. 88 patients who received neo-adjuvant chemotherapy and 140 patients who received surgery alone were investigated using a tissue microarray approach.
Results
Calpain-1, calpain-2 and calpastatin expression was associated with adverse cancer-specific survival in the neo-adjuvant cohort (P = 0.004, P = 0.001 and P = 0.012 respectively); which remained significant in multivariate analysis (Hazard ratio (HR) = 0.337; 95 % confidence interval (CI) = 0.140–0.81; P = 0.015, HR = 0.375; 95 % CI = 0.165–0.858; P = 0.020 and HR = 0.481; 95 % CI = 0.257–0.900; P = 0.022 respectively). Calpain-1 and calpastatin expression was also associated with adverse cancer specific survival in the primary surgery cohort (P = 0.001 and P = 0.013 respectively); which remained significant in multivariate analysis (HR = 0.309; 95 % CI = 0.159–0.601; P = 0.001 and HR = 0.418; 95 % CI = 0.205–0.850; P = 0.016 respectively). Calpain-9 expression was not associated with cancer-specific survival in the neo-adjuvant and primary surgery cohorts.
Conclusion
Determining the expression levels of calpain-1, calpain-2 and calpastatin may provide clinically relevant prognostic information for gastro-oesophageal adenocarcinomas; these findings warrant further studies in larger cohorts of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric and oesophageal cancer represents a significant portion of the total worldwide cancer burden. Operable gastro-oesophageal carcinomas are treated with radical surgery, which is critical in the management of the disease; however, clinical trials have demonstrated a reduction in the risk of death in patients given peri-operative chemotherapy opposed to surgery alone [1]; furthermore, the OEO2 trial investigated surgical resection with and without pre-operative chemotherapy in oesophageal cancer has also shown a risk reduction [2]. Neo-adjuvant platinum-based chemotherapy is the standard of care for patients with gastro-oesophageal cancers, with approximately 40 % of patients responding to treatment [1, 3].
The calpain system is currently comprised of 14 known enzymes that function as calcium-dependent cysteine proteases; the most widely studied of which are micro (μ)-calpain and milli (m)-calpain, which are named for the calcium concentration required for their activation [4, 5]. μ-calpain and m-calpain are each composed of a large 80 kDa catalytic subunit [CAPN1 (calpain-1) and CAPN2 (calpain-2), respectively] and a small 28 kDa regulatory subunit (CAPNS1) which is common to both enzymes [6]. Calpastatin is the endogenous inhibitor of calpain, and as such is ubiquitously expressed. The calpain system has been implicated in many cellular functions such as cytoskeletal remodelling, cellular signalling and apoptosis, and is implicated in tumorigenesis through altered expression and activity in cancer [7]. Calpain expression is altered in a number of tumour types, including breast cancer [8, 9] and ovarian cancer [10]. μ-calpain and milli-calpain are both expressed in the gastrointestinal tract, in addition to the tissue specific calpain-8 (nCL-2) and calpain-9 (nCL-4). Calpain activity has been implicated in the response to 5-fluorouracil (5-FU) by altering the levels of thymidylate synthase and the 5-FU metabolite 5-fluoro-dUMP complex in gastric cancer in vitro, by which high calpain activity contributes to 5-FU chemoresistance [11]. Furthermore, calpain activation induced by Helicobacter pylori has been shown to disrupt epithelial adherens junctions, which is thought to influence the severity of disease and which is a risk factor for developing gastric adenocarcinoma [12]. Decreased expression of CAPN9 (calpain-9) has been observed in gastric cancer [13], and a complex of calpain-8 and calpain-9 (G-calpain) has been implicated in mucosal defence [14].
The aim of this study was to investigate the expression of calpain-1, calpain-2, calpain-9 and calpastatin in gastro-oesophageal cancers, in particular in those patients who received surgery alone and those patients exposed to neo-adjuvant platinum based chemotherapy prior to surgery, to determine if calpain system expression is associated with clinical outcome or clinicopathological variables.
Methods
Clinical samples
This study is reported according to REMARK criteria [15]. Tissue was obtained from patients treated at Nottingham University Hospitals Trust between 2001 and 2008 and approved by the local ethics committee of Nottingham University Hospitals. The study cohort consisted of 140 gastric/gastro-oesophageal cancer patients who received surgery and adjuvant chemotherapy, and 88 patients with operable cases of gastro-oesophageal cancer who received at least one cycle of pre-operative platinum based neo-adjuvant chemotherapy. Patients who received neo-adjuvant chemotherapy were treated with neo-adjuvant ECF chemotherapy [epirubicin (50 mg/m2), cisplatin (60 mg/m2) and continuous infusional 5-FU (200 mg/m2 per day)] or ECX chemotherapy [epirubicin (50 mg/m2), cisplatin (60 mg/m2) and capecitabine (625 mg/m2 p.o. b.d continuously)], for up to three cycles prior to surgery, or CF chemotherapy (cisplatin (80 mg/m2) and infusional 5-FU (1000 mg/m2 daily for 4 days)) for up to two cycles prior to surgery. Three patients had either carbo/F or CX.
The median follow up for the neo-adjuvant cohort was 28.4 months, and the median time to recurrence was 9.1 months; for the primary surgery cohort the median follow-up time was 27.3 months, and the median time to recurrence was 10.2 months. Disease specific survival was calculated from the date of diagnosis until 26th November 2010, when any remaining survivors were censored. Tumour regression grade (TRG) was defined as per Mandard’s criteria [16]. Table 1 shows the clinicopathological characteristics of the patient cohort.
Tissue microarray and immunohistochemistry
The tissue microarray (TMA) was prepared using triplicate 0.6 mm tissue cores of tumour, identified by a specialist pathologist, placed into a single recipient paraffin block. 4 μm sections of the TMA were mounted on poly-l-lysine coated slides. Immunohistochemistry was performed on the TMA slides which were initially deparaffinised in xylene followed by rehydration through a decreasing concentration of ethanol. Antigen retrieval was performed in 0.01 mol L−1 sodium citrate buffer (pH 6) in a microwave; 450 W for 10 min. Endogenous peroxidase activity was blocked over 10 min in 0.01 % hydrogen peroxide in methanol. Primary antibodies; mouse anti-calpastatin (1:15,000), mouse anti-calpain-1 (1:2500), rabbit anti-calpain-2 (1:2500) (all Chemicon, Massachusetts, USA, clones PI-11, P-6 and rabbit polyclonal AB1625 respectively with specificity confirmed by Western blotting) and calpain-9 (1:100) (Abnova, Taipei, Taiwan, clone 3A6) were diluted in blocking serum and applied to the tissue for 1 h at room temperature. Staining was achieved using the Vectastain Elite ABC kit (universal), containing blocking serum, biotinylated secondary antibody and ABC reagent (Vector Laboratories, Peterborough, UK) for calpain-1, calpain-2 and calpastatin. Immunohistochemical reactions were developed with 3,3′ diaminobenzidine as the chromogenic peroxidase substrate (Dako, Glostrup, Denmark). Sections were then counterstained with Gills formula haematoxylin (Vector Laboratories). Calpain-9 staining was achieved using Novolink Polymer Detection System (Leica Microsystems, Wetzlar, Germany). Following staining, sections were dehydrated and fixed in xylene prior to mounting with DPX. Breast tumour composite sections which comprised of 6 stage 1 breast tumours of grade 1–3 were included as positive and negative controls with each run, with the negative control having primary antibody substituted for PBS [9].
Assessment of staining was conducted after scanning of the slides with a Nanozoomer Digital Pathology Scanner (Hamamatsu Photonics) at 20× magnification. Calpastatin and calpain expression in tumour cells was manually assessed using an immunohistochemical H-score. Staining intensity was assessed as; none (0), weak (1), medium (2) and strong (3) over the percentage area of each staining intensity. H scores were calculated by multiplying the percentage area by the intensity grade (H score range 0–300). A minimum of 30 % of cores were scored by an independent assessor blinded to primary scores, clinicopathological criteria and clinical information. H-scores showed good concordance between assessors with single measure intraclass correlations greater than 0.7.
Statistical analysis
The relationship between categorised protein expression and clinicopathological variables was assessed using Pearson's chi-square (χ2) test of association. Spearman rank order correlations were performed to test for correlation between the expression level of different proteins. Survival curves were plotted according to the Kaplan–Meier method and significance determined using the log-rank test. Multivariate survival analysis was performed by Cox proportional hazards regression model. Independent Mann–Whitney U tests were performed to test the H-scores between patient cohorts. All differences were deemed statistically significant at the level of P < 0.05. Statistical analysis was performed using SPSS 19.0 software (IBM Corporation, NY, USA). Stratification cut-points were determined using X-Tile software (Yale School of Medicine, CT, USA) and were determined prior to statistical analyses [17].
Results
Staining location and frequency
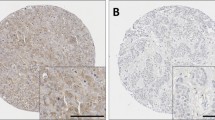
Expression of calpain-1, calpain-2, calpain-9 and calpastatin was located in the cytoplasm, with some degree of heterogeneity within samples; a limited number of specimens displayed nuclear protein expression. Typical staining patterns are shown in Fig. 1. In the neo-adjuvant cohort calpain-1 had a mean H-score of 63 and range of 184, calpain-2 had a mean H-score of 78 and a range of 255, calpain-9 had a mean H-score of 35 and a range of 165, and calpastatin has a mean H-score of 132 and a range of 290. In the primary surgery cohort, calpain-1 had a mean H-score of 96 and range of 175, calpain-2 had a mean H-score of 79 and a range of 220, calpain-9 had a mean H score of 33 and a range of 210, and calpastatin has a mean H-score of 111 and a range of 290. X-tile was used to generate cut-points for analysis; in the neo-adjuvant cohort calpain-1 had a H-score cut point of 21 with 12.9 % (11/85) of cases having low expression, calpain-2 had a cut point of 110 with 70.1 % (54/77) of cases having low expression, calpain-9 had a cut point of 35 with 63.1 % (41/75) of cases having low expression, and calpastatin had a cut point of 130 with 53.1 % (43/81) of cases having low expression. In the primary surgery cohort, calpain-1 had a H-score cut point of 102 with 50.4 % (64/127) of cases having low expression, calpain-2 had a cut point of 48 with 28.9 % (33/114) of cases having low expression, calpain-9 has a cut point of 45 with 29.8 % (34/114) of cases having low expression, and calpastatin had a cut point of 51 with 26.3 % (31/118) of cases having low expression. The expression of each protein in both the neo-adjuvant and the primary surgery cohorts is shown in Fig. 2; calpain-1 and calpain-9 expression was significantly different between the two patient cohorts (P < 0.001), however no significant difference was observed in the expression of calpain-2 and calpastatin between patient cohort.
Representative photomicrographs of high and low levels of expression. a is high calpain-1 and b is low calpain-1 expression. c is high calpain-2 and d is low calpain-2 expression. e is high calpastatin and f is low calpastatin expression. g is high calpain-9 expression and h is low calpain-9 expression photomicrographs are at ×10 magnification with ×20 magnification inset box where scale bar shows 100 μm
In the neo-adjuvant cohort, the expression of calpain-1 was not correlated with expression of calpain-2 (r = 0.169; P = 0.158) or expression of calpastatin (r = 0.170; P = 0.133); however calpain-2 expression did correlate with calpastatin expression (r = 0.537; P < 0.001). In the primary cohort calpain-1 expression was not correlated with calpain-2 expression (r = 0.071; P = 0.425), but did correlate with expression of calpastatin (r = 0.341; P < 0.001). In addition, calpain-2 expression was correlated with calpastatin expression in this cohort (r = 0.331; P < 0.001).
Relationship with clinicopathologic criteria
The expression of members of the calpain system was assessed for association with clinicopathological variables, which are shown in Tables 1 and 2. In the neo-adjuvant cohort, high calpain-1 expression was associated with the presence of perineural invasion (χ2 = 22.793; degrees of freedom (df) = 1; P < 0.001) and high calpain-2 expression was associated with the site of the tumour (χ2 = 7.640; df = 2; P = 0.022). High calpastatin expression was associated with low tumour category (χ2 = 6.3.02; df = 1; P = 0.012), lymph node negative disease (χ2 = 6.841; df = 1; P = 0.009), and lower overall tumour category (χ2 = 5.343; df = 1; P = 0.021). In the primary surgery cohort high calpain-1 expression was associated with the site of the tumour (χ2 = 11.855; df = 1; P < 0.001) and lymph node negative tumours (χ2 = 3.992; df = 1; P = 0.046); calpain-2 expression was associated with the site of the tumour (Fisher's exact P = 0.033). Calpain-9 and calpastatin expression was not associated with any clinicopathological variables.
Relationship with clinical outcome
The expression of calpain-1, calpain-2 and calpastatin was tested for association with clinical outcome in both the neo-adjuvant and primary surgery cohorts. In the neo-adjuvant cohort, low calpain-1, calpain-2 and calpastatin expression was associated with adverse cancer-specific survival (P = 0.004, P = 0.001 and P = 0.012 respectively) (Fig. 3). In multivariate analysis, including tumour category, node category, overall stage, vascular invasion, and TRG status, calpain-1 calpain-2 and calpastatin expression remained significant for cancer-specific overall survival (hazard ratio (HR) = 0.337; 95 % confidence interval (CI) = 0.140–0.81; P = 0.015, HR = 0.375; 95 % CI = 0.165–0.858; P = 0.020 and HR = 0.481; 95 % CI = 0.257–0.900; P = 0.022, respectively) (Table 3). If calpain-1, calpain-2 and calpastatin expression are included in the multivariate analysis against the same clinicopathologic criteria, only calpain-1 remains significant (HR = 0.281; 95 % CI = 0.106–0.741; P = 0.010) (Table 3). In the primary surgery cohort low calpain-1 and calpastatin expression was associated with adverse cancer specific survival (P = 0.001 and P = 0.013, respectively) (Fig. 4). In multivariate analysis including tumour category, node category, overall stage, vascular invasion, perineural invasion and TRG status, both calpain-1 and calpastatin expression remained significant for cancer-specific overall survival (HR = 0.309; 95 % CI = 0.159–0.601; P = 0.001 and HR = 0.418; 95 % CI = 0.205–0.850; P = 0.016, respectively) (Table 4). If calpain-1 and calpastatin expression are included in the multivariate analysis against the same clinicopathologic criteria, only calpain-1 remains significant (HR = 0.304; 95 % CI = 0.140–0.657; P = 0.002) (Table 4). Calpain-9 expression was not significantly associated with adverse cancer-specific survival in both neo-adjuvant (P = 0.184) and primary surgery (P = 0.426) cohorts.
Kaplan–Meier analysis of cancer-specific overall survival showing the impact of calpain-1 (panel a), calpain-2 (panel b) and calpastatin (panel c) expression in the cohort of gastro-oesophageal cancer patients treated with neo-adjuvant chemotherapy with significance determined using the log rank test. The numbers shown below the Kaplan–Meier survival curves are the number of patients at risk at the specified month. Grey lines denote low protein expression, and black lines denote high protein expression. Panel a: low-expression group has 9 observations and 7 events and high-expression group has 66 observations and 39 events; panel b: low-expression group has 44 observations and 32 events and high-expression group has 23 observations and 11 events; panel c: low-expression group has 37 observations and 30 events and high-expression group has 34 observations and 16 events; where observations are the number of observations per group and events are those patients who died of their disease
Kaplan–Meier analysis of cancer-specific overall survival showing the impact of calpain-1 (panel a), calpain-2 (panel b) and calpastatin (panel c) expression in the cohort of gastro-oesophageal cancer patients treated with primary surgery with significance determined using the log rank test. The numbers shown below the Kaplan–Meier survival curves are the number of patients at risk at the specified month. Grey lines denote low protein expression, and black lines denote high protein expression. Panel a: low-expression group has 41 observations and 31 events and high-expression group has 43 observations and 17 events; panel b: low-expression group has 19 observations and 8 events and high-expression group has 57 observations and 34 events; panel c: low-expression group has 19 observations and 15 events and high-expression group has 61 observations and 30 events; where observations are the number of observations per group and events are those patients who died of their disease
Discussion
Neo-adjuvant chemotherapy has been shown to reduce the risk of death in gastro-oesophageal cancer; however there are a number of patients that will not respond to chemotherapy who would have otherwise received surgery sooner [1]. Furthermore, the efficacy of adjuvant chemotherapy in patients who do not respond to neo-adjuvant chemotherapy is unclear. The expression levels of calpain-1, calpain-2, calpain-9 and calpastatin was investigated in gastro-oesophageal cancers that were separated into two cohorts; one treated with at least one cycle of pre-operative platinum-based neo-adjuvant chemotherapy and one cohort receiving primary surgery. The expression of calpain was determined in both cohorts due to the large difference in treatment regimens and to correspond with current clinical practice; furthermore, calpain activity has been implicated in the in vitro response to 5-FU in gastric cancer [11].
Low calpain-1 expression was associated with adverse cancer-specific survival in both the neo-adjuvant and primary surgery cohorts (P = 0.004 and P = 0.001, respectively). This remained significant in multivariate analysis including potential confounding factors (P = 0.015 and P = 0.001, respectively). This finding suggests that calpain-1 expression could be used as a prognostic marker in patients treated by surgery with or without neo-adjuvant chemotherapy. Interestingly, it is low expression that is associated with adverse clinical outcome; this is the opposite of observations made in breast cancer [8] and ovarian cancer [10], and it may be that the effect of calpain expression is tumour-type specific. Similar observations were made for low calpastatin expression and adverse cancer-specific survival in both the neo-adjuvant and primary surgery cohorts (P = 0.012 and P = 0.013, respectively) which also remained significant in multivariate analysis (P = 0.022 and P = 0.016, respectively). Perhaps most interestingly, low calpain-2 expression was significantly associated with adverse cancer-specific survival in the neo-adjuvant cohort (P = 0.001), which remained significant in multivariate analysis (P = 0.020), but was not associated with cancer-specific survival in the primary surgery cohort. No associations between calpain-9 and survival were observed. It is unclear as to why low expression of calpain would be associated with poor survival in gastric carcinomas, however calpain expression is implicated in numerous cellular processes such as apoptosis and migration [7]; it may be that calpain expression is more closely linked with apoptosis in gastric cancer. Furthermore, low gene expression of CAPN9 and CAPN8 have been described in gastric cancer, although high calpain activity reduces chemosensitivity to 5-FU in gastric cancer in vitro [11, 13]. This study assessed the expression level of the calpain system, and it is important to note that determining calpain expression cannot provide any estimate of calpain activity levels. There are a small number of antibodies that are able to detect calpain-cleavage products to provide some estimation of relative enzyme activity, however these antibodies require further optimisation in formalin fixed tissue, as well as in human malignancies [18, 19].
This study describes the importance of the calpain system in the prognosis of gastric adenocarcinoma patients. In terms of the most clinically relevant protein to assess, it could be argued that calpain-1 would provide the most important prognostic information in both the neo-adjuvant and primary surgery. This is because calpain-1 is significantly associated with survival in multivariate analysis in both the neo-adjuvant and primary surgery cohorts when various clinicopathological information (P = 0.015 and P = 0.001 respectively), but also when other calpain system members are included in the multivariate model (P = 0.010 and P = 0.002, respectively).
The standard of care for gastro-oesophageal adenocarcinoma patients is neo-adjuvant chemotherapy and surgical resection. This study investigated the protein expression levels of calpain-1, calpain-2, calpain-9 and calpastatin in surgically excised tumours treated with neo-adjuvant chemotherapy or tumours that had not been previously exposed to chemotherapy to show the importance of expression levels of the calpain system. Calpain-1, calpain-2 and calpastatin may be clinically relevant prognostic biomarkers in gastro-oesophageal adenocarcinomas, and these findings warrant further studies in larger cohorts of patients.
References
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59:549–66.
Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801.
Kawasaki H, Imajoh S, Kawashima S, Hayashi H, Suzuki K. The small subunits of calcium dependent proteases with different calcium sensitivities are identical. J Biochem. 1986;99:1525–32.
Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer. 2011;11:364–74.
Storr SJ, Woolston CM, Barros FF, Green AR, Shehata M, Chan SY, et al. Calpain-1 expression is associated with relapse-free survival in breast cancer patients treated with trastuzumab following adjuvant chemotherapy. Int J Cancer. 2011;129:1773–80.
Storr SJ, Mohammed RA, Woolston CM, Green AR, Parr T, Spiteri I, et al. Calpastatin is associated with lymphovascular invasion in breast cancer. Breast. 2011;20:413–8.
Storr SJ, Safuan S, Woolston CM, Abdel-Fatah T, Deen S, Chan SY, et al. Calpain-2 expression is associated with response to platinum based chemotherapy, progression-free and overall survival in ovarian cancer. J Cell Mol Med. 2012;16(10):2422–8.
Nabeya Y, Suzuki T, Furuya A, Koide N, Ohkoshi M, Takiguchi M, et al. Calpain regulates thymidylate synthase-5-fluoro-dUMP complex levels associated with response to 5-fluorouracil in gastric cancer cells. Cancer Sci. 2011;102:1509–15.
O’Connor PM, Lapointe TK, Jackson S, Beck PL, Jones NL, Buret AG. Helicobacter pylori activates calpain via toll-like receptor 2 to disrupt adherens junctions in human gastric epithelial cells. Infect Immun. 2011;79:3887–94.
Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–63.
Hata S, Abe M, Suzuki H, Kitamura F, Toyama-Sorimachi N, Abe K, et al. Calpain 8/nCL-2 and calpain 9/nCL-4 constitute an active protease complex, G-calpain, involved in gastric mucosal defense. PLoS Genet. 2010;6:e1001040.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol. 2005;2:416–22.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–6.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Goncalves I, Nitulescu M, Saido TC, Dias N, Pedro LM, JF E Fernandes, et al. Activation of calpain-1 in human carotid artery atherosclerotic lesions. BMC Cardiovasc Disord. 2009;9:26.
Neumar RW, Meng FH, Mills AM, Xu YA, Zhang C, Welsh FA, et al. Calpain activity in the rat brain after transient forebrain ischemia. Exp Neurol. 2001;170:27–35.
Acknowledgments
The authors greatly acknowledge the Davies Memorial fund for funding SJS.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Storr, S.J., Pu, X., Davis, J. et al. Expression of the calpain system is associated with poor clinical outcome in gastro-oesophageal adenocarcinomas. J Gastroenterol 48, 1213–1221 (2013). https://doi.org/10.1007/s00535-012-0743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0743-4