Abstract
Background
Pegylated interferon (PEG-IFN) plus ribavirin (RBV) therapy is the current standard of care for patients with chronic hepatitis C. Determining precisely the risk of serious adverse events (SAEs) and mortality from a single study is rather difficult because of the infrequency of such events. The aim of this systematic review was to assess the rates of SAEs and the mortality of PEG-IFN/RBV therapy in a pooled large sample, and to assess the relationship between SAEs and mortality rates and therapeutic characteristics.
Methods
A literature search was conducted using MEDLINE, EMBASE, and the Cochrane Library to identify randomized controlled trials evaluating the efficacy and safety of PEG-IFN/RBV therapy. We calculated the crude mortality and SAE rates with 95 % confidence intervals (CIs).
Results
Eighty studies with 153 treatment arms that included 27569 patients were enrolled (14401 patients treated with Peg-IFN alpha-2a/RBV and 13168 with Peg-IFN alpha-2b/RBV). All-cause and treatment-related deaths were observed in 50 (0.18 %; 95 % confidence interval [CI] 0.13–0.24 %) and sixteen (0.058 %; 95 % CI 0.033–0.094 %) patients, respectively. The crude SAE rate was 7.08 % (95 % CI 6.75–7.41 %). Subgroup analysis revealed higher SAE rates in patients receiving PEG-IFN alpha-2a than in those with PEG-IFN alpha-2b (7.45 vs. 6.74 %), and higher SAE rates with higher doses than with the lower doses in PEG-IFN-2a and 2b (11.94 vs. 6.99 %, 7.10 vs. 5.05 %, respectively), and with extended duration (>48 weeks) than with standard duration (48 weeks) (15.5 vs. 6.67 %) in PEG-IFN alpha-2a.
Conclusion
The mortality rate during PEG-IFN/RBV therapy was acceptably low, but the rate of SAEs was not negligible in a treatment for a benign disease, and the rate was affected by treatment regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic hepatitis C virus (HCV) infection affects more than 170 million people worldwide and is a major cause of cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. Currently the standard of care for patients with chronic hepatitis C is peginterferon (PEG-IFN) plus ribavirin (RBV) therapy, which can induce a sustained virological response (SVR) in 40–50 % of treatment-naïve patients with genotype 1 and an SVR of approximately 80 % in treatment-naïve patients with genotypes 2 or 3 [3–8]. After an SVR is achieved, the risk of developing liver failure and HCC is greatly reduced [9]. However, this treatment is associated with various types of complications, some of which lead to fatal outcomes. Because death during treatment is a rare event, a large sample size is needed to accurately assess the mortality rate and risk factors. The aim of this systematic review was to assess the rates of serious adverse events (SAEs) and mortality during PEG-IFN/RBV therapy in a pooled large-sized sample and to assess the relationship between mortality and SAE rates and therapeutic characteristics.
Methods
Study search protocol
We searched MEDLINE, EMBASE, and the Cochrane Library to identify randomized controlled trials (RCTs) evaluating the efficacy and safety of PEG-IFN/RBV therapy published between December 1999 and October 2010. We used the following search terms: chronic hepatitis C, interferon, and pegylated or peg or peginterferon or pegasys or pegintron. The search was limited to the English language.
Inclusion criteria
Studies were included in the analysis if: (1) they were RCTs, (2) they included at least one PEG-IFN/RBV treatment group in patients with chronic hepatitis C, (3) they clearly specified adverse events, (4) patients were followed up until at least 24 weeks after the end of treatment, and (5) the studies had been published or accepted for publication as full-length articles. Studies were excluded if: (1) they dealt only with co-infection of HCV and HIV; (2) they dealt only with patients with a specific condition such as a comorbid disease (e.g., cryoglobulinemia), status after liver transplantation, or patients on dialysis; (3) they included patients under 18 years of age; or (4) they focused on specific adverse events or only on hemodynamic status. We restricted the included studies to RCTs on the premise that the quality of RCTs is superior to that of non-randomized or retrospective studies.
Data extraction
Two authors (T. M. and T. K.) independently screened titles and abstracts for potential eligibility and the full texts for final eligibility. Disagreements were resolved by consensus or by consulting a third author (R. T.). We extracted the data using a standardized data-collection form to record details of the study design, treatment doses and duration, number of patients in the arm, patient characteristics, and outcomes. A database using Microsoft Access 2010 (Microsoft, Redmond, WA, USA) was developed specifically for that purpose. Two authors independently entered data into the form, and the data were then compared. Any discrepancies were checked and resolved by consensus.
Statistical analysis
The primary and secondary outcome measures were mortality and SAE rates during PEG-IFN/RBV therapy, respectively. We recorded the number of SAEs and deaths observed in each arm of the included studies. We calculated crude mortality and SAE rates with 95 % confidence intervals (CI) by dividing the total number of deaths or SAEs observed by the total number of patients in the relevant group. Studies that did not discriminate serious adverse events from others were not included in the analyses of the corresponding outcome. We performed a subgroup analysis by comparing mortality and SAE rates between PEG-IFN alpha-2a and alpha-2b, high-dose and low-dose PEG-IFN, and shorter and longer treatment durations. We also performed a meta-regression analysis to investigate relationships between mortality and SAE rates and continuous variables (mean age; mean body weight; proportion of males; proportion of Caucasian, African, and Asian patients; and proportion of genotype 1 patients) using the random effects model. Heterogeneity was tested using the I 2 test to calculate the percentage of variation caused by heterogeneity rather than by chance alone [10]. The analyses were performed with S-plus Ver. 7.0 (Insightful, Seattle, WA, USA) and StatsDirect version 2.7.7 (StatsDirect, Chesire, UK). The threshold of the reported P value accepted as indicating significance was <0.05.
Results
Study characteristics
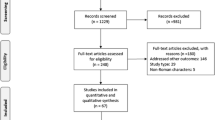
Figure 1 shows the results of the screening. Our initial database search retrieved 323 citations, of which 243 were excluded because they did not meet our inclusion criteria; therefore, a total of 80 RCTs with 153 arms that included 27569 patients were enrolled. Table 1 shows the characteristics of each enrolled study. All studies contained at least one treatment arm using PEG-IFN and RBV for chronic hepatitis C. There were 16797 male and 10254 females and the sex was not reported in the remaining 518 patients. The mean age was 45.9 years. A total of 14401 patients were treated with PEG-IFN alpha-2a/RBV, and 13168 with PEG-IFN alpha-2b/RBV. PEG-IFN alpha-2a was used at a fixed dose of 180–360 μg/body/week, and PEG-IFN alpha-2b at weight-based doses of 0.35–3.0 μg/kg/week. The RBV dose was fixed in 36 treatment arms and weight-based in 117 treatment arms. Treatment duration ranged from 12 to 72 weeks. The numbers of patients with HCV genotypes 1, 2, 3, and 4–6 were 18082, 3427, 3519, and 842, respectively. The genotype was not reported in the remaining 1699 patients. The treatment protocol is described in Supplementary Table 1.
Primary outcome
A total of 50 deaths from all causes were observed in the enrolled studies. Of these, sixteen were considered by the authors to be treatment-related. The crude overall and treatment-related mortality rates were 0.18 % (95 % CI 0.13–0.24 %) and 0.058 % (0.033–0.094 %), respectively. There was no evidence of heterogeneity among studies for mortality and treatment-related mortality (I 2 = 0 in both). The causes of mortality were suicide (N = 6), drug intoxication (N = 6), myocardial infarction (N = 3), sepsis (N = 3), aortic dissection (N = 2), traffic accident (N = 2), HCC (N = 2), rupture of a cerebral aneurysm (N = 1), bronchitis (N = 1), syncope (N = 1), pulmonary tuberculosis (N = 1), and unknown causes (N = 22). The six cases of suicide, the three of sepsis, two of drug intoxication, two of myocardial infarction, one of HCC, one of syncope, and one of pulmonary tuberculosis were considered by the investigators to be treatment-related mortality.
Subgroup analysis did not reveal any difference in mortality according to type and dose of PEG-IFN or in relation to duration of treatment (Fig. 2).
Secondary outcome
Seventy-two studies with 135 treatment arms including 23996 patients reported SAEs. They reported SAEs such as anemia requiring transfusion, neutropenia below 500/mm3, hypothyroidism, psychosis, pneumonia, and cellulitis. SAEs were not discriminated from other adverse events in the remaining eighteen studies. The crude SAE rate was 7.08 % (95 % CI 6.75–7.41 %). Significant heterogeneity among studies was found for this outcome (I 2 = 94.1 %). SAE rates were higher in PEG-IFN alpha-2a than in PEG-IFN alpha-2b (7.45 vs. 6.74 %). In a subgroup analysis of the type and dose of PEG-IFN and duration of treatment, higher SAE rates were observed for intensive (270–360 μg) than for standard (180 μg) doses and for extended (>48 weeks) than for standard (48 weeks) treatment duration in patients treated with PEG-IFN alpha-2a, and for standard (1.5 μg/kg) than for lower (≤1.0 μg/kg) doses in patients treated with PEG-IFN alpha-2b (Fig. 3). However, heterogeneity remained evident in all subgroups.
Meta-regression analysis showed that greater body weight, an increased proportion of male patients, an increased proportion of HCV genotype 1, and an increased proportion of Caucasian patients and decreased proportion of Asian patients were significantly associated with increased SAE rates (Table 2). There was no significant association between increased SAE rates and the mean patient age or the proportion of African patients.
Discussion
According to a report by the World Health Organization (WHO), age-specific annual death rates from all causes in individuals aged 45–49 years in the United States, Italy, and Japan (locations of the majority of our enrolled studies) were 409, 216, and 248 per 100000, respectively [11]. The crude mortality of 0.18 % (180 per 100000) found in the present study is low by comparison, even allowing for the biased population tolerable to PEG-IFN/RBV. Furthermore, the annual mortality rate could have been lower than the crude mortality rate, considering that the study period was longer than 1 year (including the follow-up period) in most enrolled studies. The annual treatment-related mortality rate could have been lower than our finding of a treatment-related mortality of 0.06 %. However, the treatment-related mortality rate may be an underestimate, as assessment of the causal relationship between treatment and mortality can be subjective and/or biased. Nonetheless, these PEG-IFN/RBV-related mortality rates would be acceptable considering the high SVR rates and considering that SVR drastically reduces adverse events related to chronic hepatitis C infection. In the present study, the most common cause of mortality was suicide, and all of the suicides were considered as treatment-related. This finding should alert treating physicians when they are treating patients with a history of psychiatric illness.
Two types of PEG-IFNs (i.e., PEG-IFN alpha-2a and 2b) are approved for the treatment of chronic hepatitis C. PEG-IFN alpha-2a has a molecular mass of 40 kDa and PEG-IFN alpha-2b a mass of 12 kDa. In comparison with PEG-IFN alpha-2b, PEG-IFN alpha-2a is less effectively cleared by the kidneys and therefore has a longer half-life. In fact, pharmacokinetic analysis in 22 patients showed that PEG-IFN alpha-2a was still detectable in 10 patients 168 h after the administration of 180 μg/week, whereas the administration of 1.0 μg/kg/week of PEG-IFN alpha-2b was undetectable in 11 of 12 patients at the same time point [12]. PEG-IFN alpha-2a is thought to be more effective than PEG-IFN alpha-2b because of its longer half-life. A recent meta-analysis showed a higher SVR rate after treatment with PEG-IFN alpha-2a than after treatment with PEG-IFN alpha-2b [13]. On the other hand, the half-life of each PEG-IFN may be related to its safety profile. However, among studies that have directly compared the safety of the two PEG-IFNs, only one reported a significant difference between SAE rates for PEG-IFN alpha-2a and PEG-IFN alpha-2b (11.7 vs. 8.6 %, P = 0.02) [14–18]. The inability of the other studies to detect such a difference may have been due to small sample sizes. In fact, a difference in SAE rates between the two PEG-IFNs was observed in pooled samples in our study.
Increasing the dose intensity of PEG-IFN and prolonging treatment duration have been attempted to achieve higher IFN levels in blood for longer periods, eventually resulting in a higher SVR rate. Treatment dose and duration are also expected to be related to the safety profile. The higher SAE rates in regimens with more intensive dosing observed for PEG-IFN alpha-2a and 2b and longer treatment duration observed for PEG-IFN alpha-2a support this hypothesis. The higher SAE rates in regimens with longer treatment duration were not observed for PEG-IFN alpha-2b, probably due to small sample sizes in regimens with longer treatment duration of PEG-IFN alpha-2b.
As mortality and SAE during PEG-IFN/RBV treatment are rare, most studies reported no such events. Therefore, the proportion calculated using the DerSimonian and Laird weight for the random-effect model showed considerable discrepancies between crude and pooled rates. In fact, pooled and treatment-related mortalities calculated using the random-effects (DerSimonian and Laird) model were 0.30 % (0.24–0.37 %) and 0.17 % (0.12–0.22 %), respectively, which were considerably different from the crude rates of each outcome (data not shown). Thus, we adopted crude instead of pooled rates for mortality and SAE.
Our meta-regression analysis showed a significant association between increased SAEs and HCV genotype 1. It is plausible that patients with genotype 1, which is difficult to treat, received a higher dose and longer duration of treatment. This is consistent with the results of the subgroup analysis.
A significant positive association between the SAE rate and the proportion of Caucasian patients, and an inverse relationship between SAEs and the proportion of Asian patients were also observed. This result may suggest a role of genetic diversity in the mechanisms underlying the adverse effects of PEG-IFN/RBV. Indeed, inosine triphosphate pyrophosphatase (ITPA) gene variants are associated with RBV-induced hemolytic anemia, and genetic polymorphisms near the interleukin-28B (IL-28B) gene were reported to be associated with response to HCV treatment with PEG-IFN and RBV, and the frequency of the variants differed between ethnic groups [19, 20].
We found that greater body weight was associated with a higher SAE rate. Of note, in the PEG-IFN alpha-2a-based regimen, the starting dose was fixed regardless of body weight; thus, with the PEG-IFN alpha-2a regimen, there might have been an overdose for patients of lower weight, leading to SAEs. However, whether such overdosing occurred was not clear in this study because there was a positive correlation between body weight and the SAE rate in patients receiving the PEG-IFN alpha-2a regimen. The reason for this positive relationship remains unclear; however, it may be because obesity is itself associated with various medical comorbidities.
We also found that an increased proportion of male patients in a study was associated with a higher SAE rate. It has been reported that female gender was an independent factor contributing to severe anemia [21], so the reason for the present finding of the increased proportion of male patients remains unclear; it may be correlated with increased body weight which caused a higher SAE rate. However, whether the proportions of individuals with obesity differed between male and female patients is not clear, because data on body mass index was often lacking.
In the present study increased mean age was not associated with a higher SAE rate, whereas discontinuation, dose reduction, and grade 3 adverse events were more frequent in older patients in previous studies [22, 23]. The lack of an association between mean age and the SAE rate in the present study could be due in part to the patients’ mean age of 45.9 years, and the proportion of patients over 60 being small. Low-risk patients tend to be included in RCTs. This is one of the limitations of this study.
Recently, the use of HCV nonstructural 3/4A serine protease inhibitors combined with PEG-IFN and RBV were reported to achieve higher SVR rates in genotype 1 patients compared with conventional PEG-IFN/RBV. These triple therapies are considered to be the next standard of care for chronic hepatitis C [24, 25]. Adverse events during triple therapies could include those related to PEG-IFN/RBV, as these regimens include PEG-IFN/RBV.
We extracted only RCTs for our analysis in order to obtain highly reliable data and minimize the influence of recall bias because RCTs are prospectively designed, and SAEs should be defined a priori. However, several limitations are still worth noting. The latent limitation of this study is inter-study variability in the definition of SAE. The precise meaning of ‘serious’ has not been determined, and some discrepancies between studies exist. These discrepancies may diminish the accuracy of the pooled SAE rate in this study. Second, even by choosing only RCTs, we could not completely exclude the influence of publication bias.
Overall, PEG-IFN/RBV treatment is relatively safe, with low mortality, considering the fact that chronic hepatitis C patients carry a high risk of cirrhosis and HCC. Nevertheless, the SAE rate with this treatment is not negligible and the development of safer regimens should be, and is, encouraged.
Abbreviations
- CI:
-
Confidence interval
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
- PEG-IFN:
-
Pegylated interferon
- RCT:
-
Randomized controlled trial
- RBV:
-
Ribavirin
- SAE:
-
Serious adverse event
- SVR:
-
Sustained virological response
- WHO:
-
World Health Organization
References
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–38.
Yvan H, Mary EK, Gregory JD, Joseph FP, Gregory LA, Geoffrey D, et al. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44(1):20–9.
Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alpha-2b plus ribavirin compared with interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alpha-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82.
Bruno S, Camma C, Di Marco V, Rumi M, Vinci M, Camozzi M, et al. Peginterferon alpha-2b plus ribavirin for naive patients with genotype 1 chronic hepatitis C: a randomized controlled trial. J Hepatol. 2004;41(3):474–81.
Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–55.
Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, et al. Peginterferon alpha-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352(25):2609–17.
Brok J, Gluud LL, Gluud C. Meta-analysis: ribavirin plus interferon vs. interferon monotherapy for chronic hepatitic C—an updated Cochrane review. Aliment Pharmacol Ther. 2010;32(7):840–50.
Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131(3):174–81.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
World Health Organization. Available at: http://apps.who.int/ghodata/?vid=720 (2011). Accessed 24 Oct 2011.
Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, et al. Viral dynamics and pharmacokinetics of peginterferon alpha-2a and peginterferon alpha-2b in naive patients with chronic hepatitis C: a randomized, controlled study. Antivir Ther. 2004;9(4):491–7.
Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alpha-2b in chronic hepatitis C: systematic review of randomized trials. Hepatology. 2010;51(4):1176–84.
Ascione A, De Luca M, Tartaglione MT, Lampasi F, Di Costanzo GG, Lanza AG, et al. Peginterferon alpha-2a plus ribavirin is more effective than peginterferon alpha-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology. 2010;138(1):116–22.
McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alpha-2b or alpha-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580–93.
Rumi MG, Aghemo A, Prati GM, D’Ambrosio R, Donato MF, Soffredini R, et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology. 2010;138(1):108–15.
Scotto G, Fazio V, Fornabaio C, Tartaglia A, Di Tullio R, Saracino A, et al. Peg-interferon alpha-2a versus Peg-interferon alpha-2b in nonresponders with HCV active chronic hepatitis: a pilot study. J Interferon Cytokine Res. 2008;28(10):623–9.
Yenice N, Mehtap O, Gumrah M, Arican N. The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turk J Gastroenterol. 2006;17(2):94–8.
Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464(7287):405–8.
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401.
Hung CH, Lee CM, Lu SN, Wang JH, Chen CH, Hu TH, et al. Anemia associated with antiviral therapy in chronic hepatitis C: incidence, risk factors, and impact on treatment response. Liver Int. 2006;26(9):1079–86.
Iwasaki Y, Ikeda H, Araki Y, Osawa T, Kita K, Ando M, et al. Limitation of combination therapy of interferon and ribavirin for older patients with chronic hepatitis C. Hepatology. 2006;43(1):54–63.
Oze T, Hiramatsu N, Yakushijin T, Mochizuki K, Oshita M, Hagiwara H, et al. Indications and limitations for aged patients with chronic hepatitis C in pegylated interferon alpha-2b plus ribavirin combination therapy. J Hepatol. 2011;54(4):604–11.
McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–38.
Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
Abergel A, Hezode C, Leroy V, Barange K, Bronowicki JP, Tran A, et al. Peginterferon alpha-2b plus ribavirin for treatment of chronic hepatitis C with severe fibrosis: a multicentre randomized controlled trial comparing two doses of peginterferon alpha-2b. J Viral Hepat. 2006;13(12):811–20.
Alfaleh FZ, Hadad Q, Khuroo MS, Aljumah A, Algamedi A, Alashgar H, et al. Peginterferon alpha-2b plus ribavirin compared with interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C in Saudi patients commonly infected with genotype 4. Liver Int. 2004;24(6):568–74.
Andriulli A, Cursaro C, Cozzolongo R, Iacobellis A, Valvano MR, Mangia A, et al. Early discontinuation of ribavirin in HCV-2 and HCV-3 patients responding to Peg-interferon alpha-2a and ribavirin. J Viral Hepat. 2009;16(1):28–35.
Angelico M, Koehler-Horst B, Piccolo P, Angelico F, Gentile S, Francioso S, et al. Peginterferon alpha-2a and ribavirin versus peginterferon alpha-2a monotherapy in early virological responders and peginterferon alpha-2a and ribavirin versus peginterferon alpha-2a, ribavirin and amantadine triple therapy in early virological nonresponders: the SMIEC II trial in naive patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2008;20(7):680–7.
Benhamou Y, Afdhal NH, Nelson DR, Shiffman ML, Halliman DG, Heise J, et al. A phase III study of the safety and efficacy of viramidine versus ribavirin in treatment-naive patients with chronic hepatitis C: ViSER1 results. Hepatology. 2009;50(3):717–26.
Berg C, Goncales FL Jr, Bernstein DE, Sette H Jr, Rasenack J, Diago M, et al. Re-treatment of chronic hepatitis C patients after relapse: efficacy of peginterferon-alpha-2a (40 kDa) and ribavirin. J Viral Hepat. 2006;13(7):435–40.
Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alpha-2a plus ribavirin. Gastroenterology. 2006;130(4):1086–97.
Berg T, Weich V, Teuber G, Klinker H, Moller B, Rasenack J, et al. Individualized treatment strategy according to early viral kinetics in hepatitis C virus type 1-infected patients. Hepatology. 2009;50(2):369–77.
Bosques-Padilla F, Trejo-Estrada R, Campollo-Rivas O, Cortez-Hernandez C, Dehesa-Violante M, Maldonado-Garza H, et al. Peginterferon alpha-2a plus ribavirin for treating chronic hepatitis C virus infection: analysis of Mexican patients included in a multicenter international clinical trial. Ann Hepatol. 2003;2(3):135–9.
Brady DE, Torres DM, An JW, Ward JA, Lawitz E,Harrison SA. Induction pegylated interferon alpha-2b in combination with ribavirin in patients with genotypes 1 and 4 chronic hepatitis C: a prospective, randomized, multicenter, open-label study. Clin Gastroenterol Hepatol. 2010;8(1):66–71e1.
Brandao C, Barone A, Carrilho F, Silva A, Patelli M, Caramori C, et al. The results of a randomized trial looking at 24 weeks vs. 48 weeks of treatment with peginterferon alpha-2a (40 kDa) and ribavirin combination therapy in patients with chronic hepatitis C genotype 1. J Viral Hepat. 2006;13(8):552–9.
Bressler B, Wang K, Grippo JF, Heathcote EJ. Pharmacokinetics and response of obese patients with chronic hepatitis C treated with different doses of PEG-IFN alpha-2a (40 kD) (PEGASYS). Br J Clin Pharmacol. 2009;67(3):280–7.
Bronowicki JP, Ouzan D, Asselah T, Desmorat H, Zarski JP, Foucher J, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alpha-2a plus ribavirin. Gastroenterology. 2006;131(4):1040–8.
Carr C, Hollinger FB, Yoffe B, Wakil A, Phillips J, Bzowej N, et al. Efficacy of interferon alpha-2b induction therapy before retreatment for chronic hepatitis C. Liver Int. 2007;27(8):1111–8.
Ciancio A, Picciotto A, Giordanino C, Smedile A, Tabone M, Manca A, et al. A randomized trial of pegylated-interferon-alpha2a plus ribavirin with or without amantadine in the re-treatment of patients with chronic hepatitis C not responding to standard interferon and ribavirin. Aliment Pharmacol Ther. 2006;24(7):1079–86.
Dalgard O, Bjoro K, Ring-Larsen H, Bjornsson E, Holberg-Petersen M, Skovlund E, et al. Pegylated interferon alpha and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology. 2008;47(1):35–42.
Diago M, Crespo J, Olveira A, Perez R, Barcena R, Sanchez-Tapias JM, et al. Clinical trial: pharmacodynamics and pharmacokinetics of re-treatment with fixed-dose induction of peginterferon alpha-2a in hepatitis C virus genotype 1 true non-responder patients. Aliment Pharmacol Ther. 2007;26(8):1131–8.
Ferenci P, Formann E, Laferl H, Gschwantler M, Hackl F, Brunner H, et al. Randomized, double-blind, placebo-controlled study of peginterferon alpha-2a (40 kD) plus ribavirin with or without amantadine in treatment-naive patients with chronic hepatitis C genotype 1 infection. J Hepatol. 2006;44(2):275–82.
Ferenci P, Brunner H, Laferl H, Scherzer TM, Maieron A, Strasser M, et al. A randomized, prospective trial of ribavirin 400 mg/day versus 800 mg/day in combination with peginterferon alpha-2a in hepatitis C virus genotypes 2 and 3. Hepatology. 2008;47(6):1816–23.
Ferenci P, Laferl H, Scherzer TM, Maieron A, Hofer H, Stauber R, et al. Peginterferon alpha-2a/ribavirin for 48 or 72 weeks in hepatitis C genotypes 1 and 4 patients with slow virologic response. Gastroenterology. 2010;138(2):503–12e1.
Fried MW, Jensen DM, Rodriguez-Torres M, Nyberg LM, Di Bisceglie AM, Morgan TR, et al. Improved outcomes in patients with hepatitis C with difficult-to-treat characteristics: randomized study of higher doses of peginterferon alpha-2a and ribavirin. Hepatology. 2008;48(4):1033–43.
Gish RG, Arora S, Rajender Reddy K, Nelson DR, O’Brien C, Xu Y, et al. Virological response and safety outcomes in therapy-naive patients treated for chronic hepatitis C with taribavirin or ribavirin in combination with pegylated interferon alpha-2a: a randomized, phase 2 study. J Hepatol. 2007;47(1):51–9.
Glue P, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, et al. A dose-ranging study of pegylated interferon alpha-2b and ribavirin in chronic hepatitis C. The Hepatitis C Intervention Therapy Group. Hepatology. 2000;32(3):647–53.
Hasan F, Al-Khaldi J, Asker H, Al-Ajmi M, Owayed S, Varghese R, et al. Peginterferon alpha-2b plus ribavirin with or without amantadine [correction of amantidine] for the treatment of non-responders to standard interferon and ribavirin. Antivir Ther. 2004;9(4):499–503.
Helbling B, Jochum W, Stamenic I, Knopfli M, Cerny A, Borovicka J, et al. HCV-related advanced fibrosis/cirrhosis: randomized controlled trial of pegylated interferon alpha-2a and ribavirin. J Viral Hepat. 2006;13(11):762–9.
Herrine SK, Brown RS Jr, Bernstein DE, Ondovik MS, Lentz E, Te H. Peginterferon alpha-2a combination therapies in chronic hepatitis C patients who relapsed after or had a viral breakthrough on therapy with standard interferon alpha-2b plus ribavirin: a pilot study of efficacy and safety. Dig Dis Sci. 2005;50(4):719–26.
Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–50.
Ide T, Hino T, Ogata K, Miyajima I, Kuwahara R, Kuhara K, et al. A randomized study of extended treatment with peginterferon alpha-2b plus ribavirin based on time to HCV RNA negative-status in patients with genotype 1b chronic hepatitis C. Am J Gastroenterol. 2009;104(1):70–5.
Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC Jr, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol. 2005;100(11):2453–62.
Jacobson IM, Brown RS Jr, Freilich B, Afdhal N, Kwo PY, Santoro J, et al. Peginterferon alpha-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46(4):971–81.
Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao-Mello CE, et al. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med. 2009;150(8):528–40.
Kamal SM, El Tawil AA, Nakano T, He Q, Rasenack J, Hakam SA, et al. Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut. 2005;54(6):858–66.
Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology. 2007;46(6):1732–40.
Kawaoka T, Kawakami Y, Tsuji K, Ito H, Kitamoto M, Aimitsu S, et al. Dose comparison study of pegylated interferon-alpha-2b plus ribavirin in naive Japanese patients with hepatitis C virus genotype 2: a randomized clinical trial. J Gastroenterol Hepatol. 2009;24(3):366–71.
Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, et al. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30(3):447–54.
Kuboki M, Iino S, Okuno T, Omata M, Kiyosawa K, Kumada H, et al. Peginterferon alpha-2a (40 kD) plus ribavirin for the treatment of chronic hepatitis C in Japanese patients. J Gastroenterol Hepatol. 2007;22(5):645–52.
Lagging M, Langeland N, Pedersen C, Farkkila M, Buhl MR, Morch K, et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008;47(6):1837–45.
Langlet P, D’Heygere F, Henrion J, Adler M, Delwaide J, Van Vlierberghe H, et al. Clinical trial: a randomized trial of pegylated-interferon-alpha-2a plus ribavirin with or without amantadine in treatment-naive or relapsing chronic hepatitis C patients. Aliment Pharmacol Ther. 2009;30(4):352–63.
Lee SD, Yu ML, Cheng PN, Lai MY, Chao YC, Hwang SJ, et al. Comparison of a 6-month course peginterferon alpha-2b plus ribavirin and interferon alpha-2b plus ribavirin in treating Chinese patients with chronic hepatitis C in Taiwan. J Viral Hepat. 2005;12(3):283–91.
Liu CH, Liu CJ, Lin CL, Liang CC, Hsu SJ, Yang SS, et al. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: a multicenter, randomized controlled trial. Clin Infect Dis. 2008;47(10):1260–9.
Lodato F, Azzaroli F, Brillanti S, Colecchia A, Tame MR, Montagnani M, et al. Higher doses of peginterferon alpha-2b administered twice weekly improve sustained virological response in difficult-to-treat patients with chronic hepatitis C: results of a pilot randomized study. J Viral Hepat. 2005;12(5):536–42.
Marcellin P, Horsmans Y, Nevens F, Grange JD, Bronowicki JP, Vetter D, et al. Phase 2 study of the combination of merimepodib with peginterferon-alpha2b, and ribavirin in nonresponders to previous therapy for chronic hepatitis C. J Hepatol. 2007;47(4):476–83.
Marcellin P, Gish RG, Gitlin N, Heise J, Halliman DG, Chun E, et al. Safety and efficacy of viramidine versus ribavirin in ViSER2: randomized, double-blind study in therapy-naive hepatitis C patients. J Hepatol. 2010;52(1):32–8.
McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362(14):1292–303.
Mecenate F, Pellicelli AM, Barbaro G, Romano M, Barlattani A, Mazzoni E, et al. Short versus standard treatment with pegylated interferon alpha-2A plus ribavirin in patients with hepatitis C virus genotype 2 or 3: the cleo trial. BMC Gastroenterol. 2010;10:21.
Mendez-Navarro J, Chirino RA, Corey KE, Gorospe EC, Zheng H, Moran S, et al. A randomized controlled trial of double versus triple therapy with amantadine for genotype 1 chronic hepatitis C in Latino patients. Dig Dis Sci. 2010;55(9):2629–35.
Meyer-Wyss B, Rich P, Egger H, Helbling B, Mullhaupt B, Rammert C, et al. Comparison of two PEG-interferon alpha-2b doses (1.0 or 1.5 μg/kg) combined with ribavirin in interferon-naive patients with chronic hepatitis C and up to moderate fibrosis. J Viral Hepat. 2006;13(7):457–65.
Napoli N, Giannelli G, Antonaci A, Antonaci S. The use of different peg-interferon alpha-2b regimens plus ribavirin in HCV-1b-infected patients after rapid virological response does not affect the achievement of sustained virological response. J Viral Hepat. 2008;15(4):300–4.
Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology. 2007;46(6):1688–94.
Roberts SK, Weltman MD, Crawford DH, McCaughan GW, Sievert W, Cheng WS, et al. Impact of high-dose peginterferon alpha-2A on virological response rates in patients with hepatitis C genotype 1: a randomized controlled trial. Hepatology. 2009;50(4):1045–55.
Roffi L, Colloredo G, Pioltelli P, Bellati G, Pozzpi M, Parravicini P, et al. Pegylated interferon-alpha2b plus ribavirin: an efficacious and well-tolerated treatment regimen for patients with hepatitis C virus related histologically proven cirrhosis. Antivir Ther. 2008;13(5):663–73.
Rossignol JF, Elfert A, El-Gohary Y, Keeffe EB. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136(3):856–62.
Rustgi VK, Lee WM, Lawitz E, Gordon SC, Afdhal N, Poordad F, et al. Merimepodib, pegylated interferon, and ribavirin in genotype 1 chronic hepatitis C pegylated interferon and ribavirin nonresponders. Hepatology. 2009;50(6):1719–26.
Sanchez-Tapias JM, Diago M, Escartin P, Enriquez J, Romero-Gomez M, Barcena R, et al. Peginterferon-alpha2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131(2):451–60.
Shiffman ML, Salvatore J, Hubbard S, Price A, Sterling RK, Stravitz RT, et al. Treatment of chronic hepatitis C virus genotype 1 with peginterferon, ribavirin, and epoetin alpha. Hepatology. 2007;46(2):371–9.
Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, et al. Peginterferon alpha-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357(2):124–34.
Shiffman ML, Ghany MG, Morgan TR, Wright EC, Everson GT, Lindsay KL, et al. Impact of reducing peginterferon alpha-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132(1):103–12.
Sjogren MH, Sjogren R Jr, Lyons MF, Ryan M, Santoro J, Smith C, et al. Antiviral response of HCV genotype 1 to consensus interferon and ribavirin versus pegylated interferon and ribavirin. Dig Dis Sci. 2007;52(6):1540–7.
Sood A, Midha V, Hissar S, Kumar M, Suneetha PV, Bansal M, et al. Comparison of low-dose pegylated interferon versus standard high-dose pegylated interferon in combination with ribavirin in patients with chronic hepatitis C with genotype 3: an Indian experience. J Gastroenterol Hepatol. 2008;23(2):203–7.
Tang KH, Herrmann E, Pachiadakis I, Paulon E, Tatman N, Zeuzem S, et al. Clinical trial: individualized treatment duration for hepatitis C virus genotype 1 with peginterferon-alpha 2a plus ribavirin. Aliment Pharmacol Ther. 2008;27(9):810–9.
Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Eight-week regimen of antiviral combination therapy with peginterferon and ribavirin for patients with chronic hepatitis C with hepatitis C virus genotype 2 and a rapid virological response. Liver Int. 2009;29(1):120–5.
von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, et al. Peginterferon-alpha-2a (40 kD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129(2):522–7.
von Wagner M, Hofmann WP, Teuber G, Berg T, Goeser T, Spengler U, et al. Placebo-controlled trial of 400 mg amantadine combined with peginterferon alpha-2a and ribavirin for 48 weeks in chronic hepatitis C virus-1 infection. Hepatology. 2008;48(5):1404–11.
Yu ML, Dai CY, Lin ZY, Lee LP, Hou NJ, Hsieh MY, et al. A randomized trial of 24- vs. 48-week courses of PEG interferon alpha-2b plus ribavirin for genotype-1b-infected chronic hepatitis C patients: a pilot study in Taiwan. Liver Int. 2006;26(1):73–81.
Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56(4):553–9.
Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, et al. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47(6):1884–93.
Zeuzem S, Diago M, Gane E, Reddy KR, Pockros P, Prati D, et al. Peginterferon alpha-2a (40 kD) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterology. 2004;127(6):1724–32.
Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, et al. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43(2):250–7.
Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, et al. Efficacy of 24 weeks treatment with peginterferon alpha-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44(1):97–103.
Zeuzem S, Yoshida EM, Benhamou Y, Pianko S, Bain VG, Shouval D, et al. Albinterferon alpha-2b dosed every two or four weeks in interferon-naive patients with genotype 1 chronic hepatitis C. Hepatology. 2008;48(2):407–17.
Conflict of interest
Kazuhiko Koike has served as a speaker for MSD and Chugai Pharmaceutical Co., Ltd., and has received research funding from MSD and Chugai Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minami, T., Kishikawa, T., Sato, M. et al. Meta-analysis: mortality and serious adverse events of peginterferon plus ribavirin therapy for chronic hepatitis C. J Gastroenterol 48, 254–268 (2013). https://doi.org/10.1007/s00535-012-0631-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0631-y