Abstract
Background
Diabetes mellitus (DM) has long been recognized as a risk factor for pancreatic cancer (PaC) and recently has attracted attention as a manifestation of PaC. Diabetes is expected to be a clue for the early detection of PaC; however, no effective screening strategy has been established.
Methods
Forty diabetic patients with PaC were identified and compared with 120 diabetic patients without any malignancies. We analyzed risk factors for and early signs of PaC, focusing on the DM-onset age.
Results
As there were peaks at 40–45 years and 60–65 years in the distribution of DM-onset age, we analyzed the clinical characteristics of and risk factors for PaC according to DM-onset age: i.e., early-onset (<55 years) and late-onset (≥55 years). PaC was diagnosed within 2 years of DM onset (new-onset) in 0 % of the patients with early-onset DM, and in 33 % of those with late-onset DM. The mean duration of DM in patients with early-onset DM with PaC was longer than that in the late-onset patients (26 vs. 9 years; P < 0.01). A family history of DM (odds ratio [OR] 3.60) and use of insulin (OR 3.52) were significant risk factors in patients with early-onset DM, while the onset age of DM (OR 1.12) and multiple diabetic patients in the family (OR 6.13) were risk factors in those with late-onset DM. Body weight loss and exacerbation of DM were seen 12 months prior to PaC diagnosis in both groups.
Conclusions
Our study revealed specific risk factors for and similar early signs of PaC in early-onset and late-onset DM. Thus, we could develop a screening strategy, combining these risk factors specific for DM-onset age with early signs of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PaC) is the fifth leading cause of cancer death in Japan [1], and the fourth in the United States [2]. The pathogenesis of this neoplasm remains unclear, but some risk factors are candidates for screening, such as diabetes [3, 4], obesity [5, 6], cigarette smoking [3], a family history of PaC [3], chronic pancreatitis [3], and intraductal papillary mucinous neoplasm [7, 8].
Attempts at surveillance of high-risk individuals have been reported [9–11]. Of these risk factors, the association between PaC and diabetes mellitus (DM) has long been recognized, and new-onset DM has been a matter of great interest recently [12–14]. The prevalence of DM in PaC patients was reported to be 40 %, and half of the DM patients with PaC had new-onset DM with a duration of 2 years or less [12]. On the other hand, long-standing DM has also been reported to be a risk factor for PaC [15, 16]. Thus, two different relationships between DM and PaC are suggested: DM as a cause of, and as a consequence of, PaC.
However, these links have not been well studied and have not been used to improve screening strategies. In this case–control study of diabetic patients treated at a specialized Japanese DM institute, we evaluated risk factors for PaC, according to DM-onset age, as well as temporal changes in diabetic status and body mass index (BMI) prior to PaC diagnosis.
Methods
Cases and controls
All cases and controls were treated for DM at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan.
We identified 40 diabetic patients with a diagnosis of PaC from the medical records at the institute. As the study institute specializes in the treatment of lifestyle-related diseases, especially DM, all the PaC cases were referred to other hospitals. From the medical records of the study institute, histological diagnosis was confirmed in 26 cases, and the remaining 14 cases were confirmed to have PaC by the clinical course. The dates of diagnosis of PaC were between March, 1999 and May, 2011. As controls, 120 diabetic patients in whom no malignancies were detected between the onset of DM and their last visit to the above institute were randomly selected. The date of the last visit was between July, 2009 and May, 2011. The controls in this study were not matched for age or sex, in order to evaluate these variables as risk factors for PaC.
Data collection and definition
All patients filled in a questionnaire at their first visit. The questionnaire contained a history of smoking and alcohol intake, history of body weight, onset age of DM, history of other diseases, and history of family members’ diseases limited to second-degree relatives. Patients visited the hospital every 1–3 months. Body weight, casual plasma glucose (CPG), and HbA1c (level according to the Japanese Diabetes Society; JDS) were measured at every visit.
The diagnosis of DM was based on the JDS criteria [17]. DM was diagnosed when hyperglycemia meeting the criteria for diabetes (fasting plasma glucose of 7.0 mmol/l [126 mg/dl] or higher; plasma glucose 2 h after 75-g glucose load of 11.1 mmol/l [200 mg/dl] or higher; or CPG load of 11.1 mmol/l [200 mg/dl] or higher) on two or more occasions on separate days. DM was also diagnosed by a single plasma glucose test meeting the criteria above if one of the following three conditions co-existed: typical symptoms of diabetes, HbA1c (JDS) of 6.5 % or higher by a standardized method, or unequivocal diabetic retinopathy.
Medical records, including the questionnaires filled in at the first visit, were retrospectively reviewed. The index date was defined as the date of diagnosis of PaC in diabetic patients with pancreatic cancer (DM with PaC), and as the date of the last visit in diabetic patients without pancreatic cancer (DM w/o PaC). Drugs used at any time between the onset of DM and the index date were reviewed. Hyperlipidemia and hypertension were also evaluated as lifestyle-related diseases, and associated medications were reviewed. A diagnosis of hyperlipidemia or hypertension was based on the need for medical treatment.
Clinical profile of diabetic patients with pancreatic cancer
We analyzed the relationship between DM-onset age and DM duration, comparing patients with DM with PaC and those with DM w/o PaC. The duration of DM was defined as the time from the date of diagnosis of DM to the index date. According to the analysis described above, we classified diabetic patients with PaC into two groups by their DM-onset age: early-onset DM (<55 years), and late-onset DM (≥55 years). The characteristics of patients with DM with PaC were compared between the early-onset and late-onset DM patients.
Risk factors for pancreatic cancer in diabetic patients
Comparing DM with PaC and DM w/o PaC, we investigated risk factors for PaC in patients with early-onset and late-onset DM. Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) were calculated for each risk factor by logistic regression analysis.
Early signs of pancreatic cancer in diabetic patients
Body weight loss and DM exacerbation are frequently seen in patients with PaC. We extracted body weight, CPG, and HbA1c (JDS) data from the medical records, and compared the temporal changes in the 2 years prior to the index date in DM with PaC and DM w/o PaC.
Statistical analysis
Comparisons of nominal variables were conducted by the χ 2 test and Fisher’s exact test, and continuous variables were compared by t-test. To investigate the relationship between onset age and duration of DM, regression lines and coefficients of determination were determined by the method of least squares. In the analysis of risk factors, ORs were calculated by logistic regression analysis. We conducted multiple logistic regression analyses, using the factors that were significant in univariate analysis. In terms of temporal changes in BMI, CPG, and HbA1c (JDS), we compared the two groups at the same time points by t-test, as well as comparing different time points within each group by paired t-test. All statistical analyses were performed using JMP 9.0.0 statistical software (SAS Institute, Cary, NC, USA). Results are presented as means ± standard deviation and differences were considered significant when the P value was less than 0.05.
Results
Onset age and duration of diabetes
The characteristics of patients with DM with PaC and those with DM w/o PaC are shown in Table 1. All patients with DM with PaC had type 2 DM. Only two patients with DM w/o PaC had type 1 DM, and the others had type 2 DM. Patients with DM with PaC were older both at DM onset (53 ± 16 vs. 45 ± 12 years; P < 0.01) and at the index date (70 ± 9 vs. 65 ± 11 years; P < 0.01).
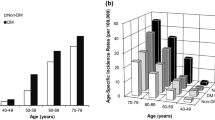
Diabetes-onset age distributions are shown in Fig. 1. Two peaks were seen in both DM with PaC (Fig. 1a) and in DM w/o PaC (Fig. 1b), although the older peak was more prominent in DM with PaC. In DM with PaC, the DM-onset age distribution had two peaks, at 40–45 years and 60–65 years. When we divided the subjects by the age of 55, which was between the 2 peaks, the proportion of late-onset DM was significantly higher in DM with PaC than in DM w/o PaC (53 vs. 27 %, P < 0.01).
The relationship between age of onset and duration of DM is shown in Fig. 2. There was a negative correlation in both DM with PaC (Fig. 2a) and in DM w/o PaC (Fig. 2b), although this correlation was stronger in DM with PaC. The r 2 values were 0.66 in DM with PaC and 0.28 in DM w/o PaC.
According to the distribution pattern of DM-onset age and the relationship between age of onset and duration of DM, we decided to classify diabetic patients with PaC into two groups by their DM-onset age: one with early-onset DM and the other with late-onset DM. The cut-off point was 55 years.
Comparison between early-onset diabetes and late-onset diabetes in patients with PaC
A comparison of early-onset DM (<55 years) and late-onset DM (≥55 years) in patients with PaC is shown in Table 2. Mean DM-onset age was 39 ± 7 years in early-onset DM, and 65 ± 9 years in late-onset DM (P < 0.01). DM duration was longer in early-onset DM (26 ± 11 years vs. 9 ± 9 years; P < 0.01), and there was no new-onset DM (within 2 years) in early-onset DM (0 vs. 33 %; P < 0.01). A family history of DM was more prevalent in early-onset DM (74 vs. 43 %; P < 0.01). More patients with early-onset DM were treated with insulin (78 vs. 40 %; P = 0.02) and sulfonylureas (94 vs. 65 %; P = 0.03).
Specific risk factors for pancreatic cancer in early-onset diabetes and late-onset diabetes
We examined risk factors for PaC in patients with early-onset and late-onset DM. The results of the univariate and multivariate analyses are shown in Table 3.
In patients with early-onset DM, a family history of DM (OR 3.60; 95 % CI 1.03–15.09; P = 0.04) and use of insulin (OR 3.52; 95 % CI 1.00–14.87; P = 0.05) were significant risk factors. Heavy smoking was associated with pancreatic cancer, but not significantly (OR 2.96; 95 % CI 0.91–10.35; P = 0.07). As to the insulin use, we further examined the relationship between the risk for PaC and the duration and the dose of insulin. The ORs were 4.77 (95 % CI 1.09–22.34; P = 0.04) in patients with a duration of insulin use of less than 5 years, and 2.47 (95 % CI 0.71–9.91; P = 0.16) in patients with a duration of 5 years or more. And the ORs were 3.50 (95 % CI 0.89–15.15; P = 0.07) in patients with a dose of less than 30 U/day, and 2.63 (95 % CI 0.72–10.81; P = 0.14) in patients with a dose of 30 U/day or more.
In patients with late-onset DM, DM-onset age (OR 1.12; 95 % CI 1.03–1.24; P < 0.01) and multiple diabetic patients in the family (OR 6.13; 95 % CI 1.20–37.91; P = 0.03) were significant risk factors. The presence of multiple cancer patients in the family was associated with pancreatic cancer, but not significantly (OR 8.10; 95 % CI 0.89–184.16; P = 0.06).
Temporal changes in BMI, CPG, and HbA1c (JDS)
Temporal changes in BMI, CPG, and HbA1c (JDS) were compared between DM with PaC and DM w/o PaC (Fig. 3). There were no significant differences in BMI (P = 0.88), CPG (P = 0.73), or HbA1c (JDS) (P = 0.52) 24 months prior to the index date between DM with PaC and DM w/o PaC.
Temporal changes in mean a body mass index (BMI), b casual plasma glucose (CPG), and c HbA1c (Japanese Diabetic Society [JDS]) in diabetic patients with and without pancreatic cancer. Bars denote ±1 standard error of the mean. DM with PaC (black circles), diabetic patients with pancreatic cancer; DM w/o PaC (white circles), diabetic patients without pancreatic cancer. The index date was defined as the date of diagnosis of pancreatic cancer in DM with PaC, and the date of the last visit in DM w/o PaC
In DM w/o PaC, BMI and CPG showed no significant changes in the 2 years before the index date. HbA1c (JDS) showed a small decrease, and compared with 24 months before the index date, the differences were significant at −21, −18, and −12 months.
In DM with PaC, BMI, compared with 24 months prior to the index date, showed a significant decrease from 12 months prior. CPG and HbA1c (JDS) showed significant increases from the same time, although the differences were not significant at −3 months for CPG, and at −9 months for HbA1c (JDS).
On comparing DM with PaC and DM w/o PaC, we found that CPG (P = 0.01) and HbA1c (JDS) (P < 0.01) were significantly higher in DM with PaC 12 months prior to the index date, but the difference in BMI was not significant (P = 0.39). At the index date, BMI was significantly lower (P = 0.01), and CPG (P < 0.01) and HbA1c (JDS) (P < 0.01) were significantly higher in DM with PaC than in DM w/o PaC. These trends were also seen in early-onset and late-onset DM.
Discussion
This case–control study of diabetic patients demonstrated two peaks in DM-onset age in DM patients with PaC, as well as showing specific risk factors for PaC in early-onset DM (<55 years) and late-onset DM (≥55 years). While treatment for DM was associated with PaC in early-onset DM, DM-onset age was a risk factor in late-onset DM. This suggests that diabetic patients with PaC comprise two subgroups. Bidirectional causality between PaC and DM has been recognized; thus, we speculate that early-onset DM with a long duration leads to PaC as a cause, and late-onset DM with a short duration arises as a consequence of PaC. Interestingly, both of these subgroups showed similar temporal changes in body weight and DM exacerbation as early signs of PaC. Thus, we could formulate different screening strategies, combining those risk factors specific to DM-onset age and common early signs.
Recent reports have emphasized new-onset DM as an early manifestation of PaC [12, 13]. However, the prevalence of PaC in new-onset DM is less than 1 % [18, 19], and it is neither cost-effective nor practical to screen all patients with new-onset DM. There have been many attempts to discover biomarkers for more effective screening [20–22], but such markers have not yet been established in the clinical setting. In the present study of patients with DM, the distribution of DM-onset age exhibited two peaks, and the age of onset and duration of DM were strongly negatively correlated in diabetic patients with PaC. We propose that the classification of diabetic patients with PaC according to age of onset of DM would lead to a better understanding of the relationship between DM and PaC. We found that no PaC developed within 2 years of the diagnosis of DM in early-onset DM, but PaC was diagnosed in 33 % of the late-onset DM group within 2 years of the diagnosis of DM.
This analysis demonstrated specific risk factors according to DM-onset age. In patients with early-onset DM, there was a median 26-year interval between DM onset and the diagnosis of PaC. Long-term hyperinsulinemia and peripheral insulin resistance can be carcinogenic [23], and treatment for DM can influence carcinogenesis. There have been many studies of the relationship between PaC risk and DM medications. Biguanides [24–26] have been reported to reduce the risk of PaC, whereas sulfonylureas [24, 26, 27] and insulins [24, 26, 27] have been reported to increase the risk. Thiazolidines have been reported to have anticancer activity [28, 29], but a cohort study reported that the use of this class of drug was not associated with the incidence of PaC [27]. In our multivariate analysis, the use of insulin was a significant risk factor for PaC only in early-onset DM. In late-onset DM, the use of DM medication was not associated with PaC. However, in our analysis of the risk of insulin use, the duration of use and the dose of insulin tended to be inversely correlated with the risk for PaC. One of the reasons for this negative correlation may be that there were patients who started to use insulin because of the exacerbation of DM caused by PaC. The ORs for PaC of long or heavy insulin users were also high, but not significant. Further investigation in a larger study population is needed to clarify the carcinogenic effect of insulin.
We also found that a combination of body weight loss and DM exacerbation were early signs of PaC in diabetic patients, with these findings being seen 12 months prior to the diagnosis of PaC. With regard to DM as an early manifestation of PaC, Pannala et al. [14] reported increased fasting blood glucose and decreased BMI in PaC patients compared with age- and sex-matched controls, and the interval between these findings and the diagnosis of PaC was 12 months. Hart et al. [30] reported body weight loss in PaC patients with new-onset DM, with a mean period of 13 months from DM onset. Our retrospective analysis showed similar trends of body weight loss and elevated CPG and HbA1c (JDS) 12 months prior to the diagnosis of PaC. The advantage of our study was the availability of detailed data on body weight, CPG, and HbA1c (JDS) every 3 months. The transient decreases in HbA1c (JDS) 9 months prior to the index date in DM with PaC might reflect intensive treatment for exacerbated DM. This trend was more prominent in early-onset DM. A retrospective review of CT images [31, 32] has shown that resectable PaC could be detected on CT scans 6–18 months prior to diagnosis; thus, PaC could be detected earlier by screening patients with DM who have risk factors and show the early signs noted above. Several attempts at PaC screening using changes in carbohydrate antigen (CA) 19-9 levels as one of the early signs of PaC have been reported, but they have resulted in limited success [33–35]. In our study, CA19-9 levels were elevated in 18 of 26 PaC patients (69 %) in whom CA19-9 levels were measured (median, 744 U/ml; range 45–5740 U/ml). However, tumor markers were not routinely measured in our study, but were measured only after PaC was strongly suspected based on clinical symptoms as well as imaging studies. In addition, there were no serial data of CA19-9 in these patients. Thus, it is difficult to discuss the importance of tumor markers for the early detection of PaC in regard to this study.
Based on the results of this study, herein we suggest a diagnostic strategy for PaC, focusing on DM according to DM-onset age (Fig. 4). In the late-onset DM patients, PaC was often diagnosed soon after the diagnosis of DM. Screening examinations, such as endoscopic ultrasonography and magnetic resonance cholangiopancreatography, should be considered when late-onset DM is diagnosed. In the late-onset DM population older age is a risk factor for PaC, so a cut-off age should be determined from the aspects of cost-effectiveness. Having multiple diabetic patients in the family is another risk factor which could be useful for selecting subjects for screening. In the early-onset DM population, PaC was diagnosed during the follow up of DM. In this population, a family history of DM and use of insulin are risk factors. Screening examinations should be considered for patients with these risk factors, especially when early signs of PaC: i.e., body weight loss and DM exacerbation, are detected.
An important limitation of our study is that the control subjects were not age- or sex-matched with patients with PaC. However, because age and sex may be risk factors for PaC, age- and sex-matched controls do not necessarily represent properly matched controls. Our study population did not include patients diagnosed with DM concomitantly with PaC, because we studied patients who were diagnosed with DM prior to the diagnosis of PaC. Gullo et al. [36] reported that about 40 % of patients with PaC had a concurrent diagnosis of DM. Other limitations of the present study are its retrospective nature and small sample size. We also lacked fasting blood glucose data, which might be more sensitive than HbA1c (JDS). CPG did not show a clear trend, probably because of a large variance resulting from diet.
In conclusion, our study confirmed the existence of two types of relationship between DM and PaC, which could be differentiated by DM-onset age. There were specific risk factors for PaC in each group. In both groups, the combination of body weight loss and DM exacerbation were early signs of PaC, and were seen 12 months prior to the PaC diagnosis. The combination of risk factors specific to DM-onset age with early signs of PaC could lead to effective screening and should be confirmed in a subsequent prospective study.
References
Ministry of Health, Labour and Welfare. Vital Statistics Japan. 2008.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464–84.
Huxley R, Ansary-Moghaddam A. Berrington de Gonzalez A, Barzi F and Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83.
Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, Obata Y, Inaba Y, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120:2665–71.
Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–8.
Tada M, Kawabe T, Arizumi M, Togawa O, Matsubara S, Yamamoto N, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265–70.
Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–5.
Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21.
Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81 (quiz 665).
Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–54.
Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101.
Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–7.
Pannala R, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318–25.
Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–9.
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202.
Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetes Res Clin Pract. 2002;55:65–85.
Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11.
Gupta S, Vittinghoff E, Bertenthal D, Corley D, Shen H, Walter LC, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4:1366–72 (quiz 1301).
Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313–8.
Basso D, Greco E, Fogar P, Pucci P, Flagiello A, Baldo G, et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin Chim Acta. 2006;372:120–8.
Pfeffer F, Koczan D, Adam U, Benz S, von Dobschuetz E, Prall F, et al. Expression of connexin26 in islets of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. Pancreas. 2004;29:284–90.
Wang F, Larsson J, Adrian TE, Gasslander T, Permert J. In vitro influences between pancreatic adenocarcinoma cells and pancreatic islets. J Surg Res. 1998;79:13–9.
Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8.
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20.
Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77.
Ferrara A, Lewis JD, Quesenberry CP Jr, Peng T, Strom BL, Van Den Eeden SK, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–9.
Galli A, Ceni E, Crabb DW, Mello T, Salzano R, Grappone C, et al. Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms. Gut. 2004;53:1688–97.
Itami A, Watanabe G, Shimada Y, Hashimoto Y, Kawamura J, Kato M, et al. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth of pancreatic cancers both in vitro and in vivo. Int J Cancer. 2001;94:370–6.
Hart PA, Kamada P, Rabe KG, Srinivasan S, Basu A, Aggarwal G, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas. 2011;40:768–72.
Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004;182:897–903.
Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–63.
Satake K, Takeuchi T, Homma T, Ozaki H. CA19-9 as a screening and diagnostic tool in symptomatic patients: the Japanese experience. Pancreas. 1994;9:703–6.
Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19–9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182–6.
Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, Badger G, et al. Screening for pancreatic cancer in a high-risk population with serum CA 19–9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87–95.
Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331:81–4.
Conflict of interest
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizuno, S., Nakai, Y., Isayama, H. et al. Risk factors and early signs of pancreatic cancer in diabetes: screening strategy based on diabetes onset age. J Gastroenterol 48, 238–246 (2013). https://doi.org/10.1007/s00535-012-0622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0622-z