Abstract
Background
Increasing evidence suggests the efficacy of interferon therapy for hepatitis C in reducing the risk of hepatocellular carcinoma (HCC). The aim of this study was to identify predictive markers for the risk of HCC incidence in chronic hepatitis C patients receiving interferon therapy.
Methods
A total of 382 patients were treated with standard interferon or pegylated interferon in combination with ribavirin for chronic hepatitis C in a single center and evaluated for variables predictive of HCC incidence.
Results
Incidence rates of HCC after interferon therapy were 6.6% at 5 years and 13.4% at 8 years. Non-sustained virological response (non-SVR) to antiviral therapy was an independent predictor for incidence of HCC in the total study population. Among 197 non-SVR patients, independent predictive factors were an average alpha-fetoprotein (AFP) integration value ≥10 ng/mL and male gender. Even in patients whose AFP levels before interferon therapy were ≥10 ng/mL, reduction of average AFP integration value to <10 ng/mL by treatment was strongly associated with a reduced incidence of HCC. This was significant compared to patients with average AFP integration values of ≥10 ng/mL (P = 0.009).
Conclusions
Achieving sustained virological response (SVR) by interferon therapy reduces the incidence of HCC in hepatitis C patients treated with interferon. Among non-SVR patients, a decrease in the AFP integration value by interferon therapy closely correlates with reduced risk of HCC incidence after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis C virus (HCV) infection is a predominant cause of liver cirrhosis and hepatocellular carcinoma (HCC) in many countries, including Japan, the United States, and countries of Western Europe [1–5]. The annual incidence of HCC in patients with HCV-related cirrhosis ranged from 1 to 8% [6–9]. Even in the absence of liver cirrhosis, patients with chronic hepatitis caused by HCV infection are at a high risk of developing HCC. Indeed, a large-scale Japanese cohort study showed that the annual incidence of HCC is 0.5% among patients with stage F0 or F1 fibrosis and 2.0, 5.3, and 7.9% among those with F2, F3, and F4 fibrosis, respectively [9]. Periodic surveillance is recommended to detect HCC as early as possible in patients with HCV-related chronic liver disease; however, this may not be cost-effective. For patients with chronic hepatitis C, more effective detection and prevention of HCC is being sought by two important routes: (1) the attempt to discover noninvasive predictive markers and (2) development of treatment strategies to reduce the risk of HCC. There have been several attempts to discover non-invasive markers capable of predicting the risk of HCC incidence in patients with chronic hepatitis C [6, 10]. For example, a cohort derived from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis (HALT-C) Trial identified older age, African American race, lower platelet count, higher alkaline phosphatase, and esophageal varices as risk factors for HCC [11].
There have also been a number of studies to evaluate the effect of anti-viral treatment of chronic hepatitis C on the incidence of HCC [12–19]. The results were summarized in a meta-analysis, which concluded that the effect of interferon on risk of HCC is mainly apparent in patients achieving a sustained virological response (SVR) to interferon therapy [13]. In addition, a number of studies have suggested the incidence of HCC is reduced in treated patients compared to historical controls [12, 15, 16, 19]. However, the recent HALT-C randomized control trial revealed that long-term pegylated interferon therapy does not reduce the incidence of HCC among patients with advanced hepatitis C who do not achieve SVRs. Reduction in the risk of HCC by maintenance therapy was shown only in patients with cirrhosis [14, 17]. These controversial results suggest that interferon therapy reduces the risk of HCC only in a group of patients with HCV-related chronic liver disease. Thus, it is important to evaluate the risk of HCC development in hepatitis C patients receiving interferon therapy and it will be clinically useful to discover markers distinguishing high- and low-risk groups.
Serum alpha-fetoprotein (AFP) has been widely used as a diagnostic marker of HCC [20–22]. However, elevation of serum AFP levels is often found in non-neoplastic liver diseases without evidence of HCC, including acute liver injury and chronic viral hepatitis [23–27], especially among patients with advanced chronic hepatitis C [28]. An increase of AFP after liver damage is interpreted as a sign of dedifferentiated hepatic regeneration [27]. There have been some reports that AFP is a significant predictor of HCC in patients with chronic hepatitis C [4, 5, 29]. In addition, it has recently been shown that AFP levels decrease in response to interferon administration in patients with chronic hepatitis C [30, 31], and that long-term interferon therapy for aged patients with chronic HCV infection is effective in decreasing serum AFP levels and preventing hepatocarcinogenesis [32, 33]. However, little is known about the relationship between changes in serum AFP level over time during interferon therapy and the development of HCC.
The aim of this large single center study was to identify predictive markers for the risk of HCC development in patients receiving interferon therapy for chronic hepatitis C. For this purpose, patients treated with standard or pegylated interferon, in combination with ribavirin, for chronic hepatitis C were enrolled and subjected to scheduled periodic surveillance for HCC and a number of potential predictive markers, including AFP and alanine aminotransferase (ALT) integration values, at a single center.
Materials and methods
Patients
Between January 2002 and April 2010, 528 patients with chronic hepatitis C received combination therapy with standard interferon and ribavirin (n = 84) or pegylated interferon and ribavirin (n = 444) at Osaka Red Cross Hospital. Eligibility criteria for treatment were positivity for serum HCV RNA and histological evidence of chronic hepatitis C (n = 427/444; 80.9%), or positivity for serum HCV RNA, liver enzyme levels greater than the normal upper limit, and an ultrasound image demonstrating chronic liver damage (n = 101/444; 19.1%). Exclusion criteria for treatment were as follows: neutrophil count <750 cells/μL, platelet count <50,000 cells/μL, hemoglobin level ≤9.0 g/dL, and renal insufficiency (serum creatinine levels >2 mg/dL).
Of 528 patients who received interferon therapy for chronic hepatitis C, 146 were excluded from this study for the following reasons: follow-up <24 weeks after the termination of the interferon therapy (n = 122), previously treated for HCC (n = 22), or occurrence of HCC during or within 24 weeks after treatment (n = 2). Therefore, 382 patients were enrolled for the study and were retrospectively analyzed.
To detect early-stage HCC, ultrasonography, dynamic contrast enhanced computed tomography (CT), dynamic contrast enhanced magnetic resonance imaging (MRI), and/or measurement of tumor markers (including AFP) were performed for all patients at least every 6 months. HCC was diagnosed radiologically as liver tumors displaying arterial hypervascularity and venous or delayed phase washout by dynamic contrast enhanced CT or MRI.
The study protocol was approved by the Ethics Committee at Osaka Red Cross Hospital and performed in compliance with the Helsinki Declaration.
Treatment protocol and definition of responses to treatment
The basic treatment protocol for patients with chronic hepatitis C consisted of 6 mega units of interferon-α-2b 3 times a week or 1.5 μg/kg of pegylated interferon α-2b once a week, combined with ribavirin at an oral dosage of 600–1000 mg/day. Duration of the treatment was 48–72 weeks for those with HCV genotype 1 and serum HCV RNA titer of >5 log IU/mL, and 24 weeks for all other patients.
Patients who were negative for serum HCV RNA for >6 months after completion of interferon therapy were defined as showing an SVR. Patients whose serum ALT levels decreased to the normal range and remained normal for >6 months after the termination of interferon therapy were defined as showing a sustained biochemical response (SBR).
Patients who did not achieve SVR received ursodeoxycholic acid and/or glycyrrhizin containing preparation (Stronger Neo-Minophagen C), when serum ALT levels were higher than the upper limit of normal.
Virological assays
HCV genotype was determined by polymerase chain reaction (PCR) amplification of the core region of the HCV genome using genotype-specific PCR primers [34]. Serum HCV RNA load was evaluated once a month during and 24 weeks after treatment using a PCR assay (Cobas Amplicor HCV Monitor, Roche Molecular Systems, Pleasanton, CA, USA).
Measurement of AFP and calculation of average integration value
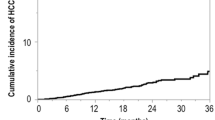
AFP was measured in serum samples obtained from each patient at intervals of 1–3 months. The median number of examinations was 15 (range 1–70) in each patient. Serum AFP levels were determined by enzyme-linked immunosorbent assay, which was performed using a commercially available kit (ELISA-AFP, International Reagents, Kobe, Japan). Integration values of AFP and ALT were calculated as described in previous reports [35]. For example, the integration value of AFP was calculated as follows, (y 0 + y 1) × x 1/2 + (y 1 + y 2) × x 2/2 + (y 2 + y 3) × x 3/2 + (y 3 + y 4) × x 4/2 + (y 4 + y 5) × x 5/2 + (y 5 + y 6) × x 6/2, i.e., the area of each trapezoid representing an AFP value was measured the sum of the resulting values used to calculate the integration value (Fig. 1). The average integration value was obtained by dividing the integration value by the observation period from initiation of the treatment.
Statistical analysis
The Kaplan–Meier method was used to estimate the rates of development of HCC in patients after interferon therapy. Log-rank tests were used to evaluate the effects of predictive factors on incidence of HCC. Significance was defined as P < 0.05. Multivariate Cox regression analysis using the stepwise method was used to evaluate the association between HCC incidence and patient characteristics, and to estimate hazard ratio (HR) with a 95% confidence interval (CI). A P value of 0.1 was used for variable selection and was regarded as statistically significant. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
Characteristics of patients and incidence of HCC
This study included 382 patients treated for chronic hepatitis C with standard interferon or pegylated interferon in combination with ribavirin. Baseline clinical and virological characteristics of patients included in the study are summarized in Table 1. The median age of the patients at the outset of therapy was 59.0 years (range 18–81 years) and the median follow-up period was 4.1 years (range 0.1–8.4 years). The majority of patients were infected with HCV genotype 1b (n = 229; 60%), and median serum HCV RNA load was 6.1 log IU/mL (range 2.3–7.3 log IU/mL). Baseline (before interferon therapy) median serum AFP level was 6.9 ng/mL (range 1.6–478.3 ng/mL).
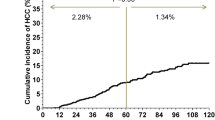
During follow-up, 23 patients (4.9%) developed HCC. The cumulative incidences of HCC, which was estimated using the Kaplan–Meier method, were 3.1, 6.6, and 13.4% at 3, 5, and 8 years, respectively (Fig. 2).
Predictive factors for incidence of HCC in all patients
Predictive factors for incidence of HCC in all 382 patients were analyzed using log-rank tests (Table 2). Univariate analysis showed that age ≥70 years (P = 0.040), non-SVR (P < 0.0001), non-SBR (P = 0.027), average ALT integration value ≥40 IU/L (P = 0.001), baseline AFP ≥10 ng/mL (P = 0.005), average AFP integration value ≥10 ng/mL (P < 0.0001), and baseline platelet count <150,000 platelets/μL (P = 0.001) were all significantly associated with the incidence of HCC. After multivariate analysis, the only variable remaining in the model was non-SVR (HR 8.413, 95% CI 1.068–66.300, P = 0.043). Further, although patients with average AFP integration values ≥10 ng/mL also appeared to have an increased risk of HCC, the difference did not reach statistical significance in the multivariate analysis (P = 0.050) (Table 3).
Predictive factors for incidence of HCC in non-SVR patients
Because non-SVR was the only predictive factor across the entire study cohort, to clarify predictive factors for incidence of HCC within this group, the same variables were further analyzed in non-SVR patients alone. By univariate analysis, average AFP integration value ≥10 ng/mL (P = 0.019) and baseline platelet count <150,000 (P = 0.0022) (Table 2) were again identified as significant predictive factors for incidence of HCC. In addition, male gender was significantly associated with incidence of HCC in non-SVR patients (P = 0.022). Multivariate analysis, however, indicated that only two variables were independently associated with incidence of HCC in non-SVR patients: average AFP integration value ≥10 ng/mL (HR 4.039, 95% CI 1.570–10.392, P = 0.004), and male gender (HR 3.636, 95% CI 1.383–9.563, P = 0.009) (Table 4). There was no significant difference in other variables including those identified as predictive factors in the entire study population (i.e., age, non-SBR, ALT integration value, AFP before interferon therapy) (Table 2).
AFP integration value as a predictive factor for HCC
Further analysis focused on the AFP integration value as this was the strongest predictive factor for incidence of HCC in non-SVR patients. Of the 382 patients, both baseline and AFP integration values were available for 321. These were divided into four groups: (1) AFP “low–low,” (2) AFP “low–high,” (3) AFP “high–low,” and (4) AFP “high–high,” for baseline AFP-average AFP integration values, respectively, where “high” is ≥10 ng/mL and “low” is <10 ng/mL. As shown in Fig. 3a, of the 321 patients, 211 (65.7%) showed baseline AFP levels <10 ng/mL. Of these 211, 207 (98%), were in the AFP low–low group, and only four in the AFP low–high groups. Baseline characteristics, including age, gender, serum HCV-RNA, aspartate aminotransferase (AST), ALT, bilirubin, white blood cell, hemoglobin, platelet, observation periods, and number of times of AFP measurement, were not different between AFP high–low group and high–high group. However, AFP-low group, which is a combination of the low–high and low–low groups, showed significantly lower AST level (P < 0.00001), lower ALT level (P < 0.00001), higher platelet count (P < 0.00001), shorter observation period (P = 0.01448), and fewer number of times of AFP examination (P = 0.00035), compared to both AFP high–high and AFP high–low group. Six patients (2.8%) with baseline AFP levels <10 ng/mL developed HCC in the follow-up period and none of these patients were among the four low–high group patients. Even in patients with high baseline AFP levels, incidence of HCC was only 3.9% among the AFP high–low group (2 of 51 patients). In contrast, 20.3% of patients in the AFP high–high group developed HCC during the follow-up period.
AFP integration value as a predictive factor for HCC. a Flow diagram showing the number of patients (n) classified by baseline alpha-fetoprotein (AFP) levels before interferon (IFN) therapy and average AFP integration value, and the incidence of hepatocellular carcinoma (HCC) of each group. b Kaplan–Meier estimates of the incidence of HCC. Solid line AFP-low group (AFP levels before interferon therapy <10 ng/mL); dotted line AFP high–low group (baseline AFP levels ≥10 ng/mL, average AFP integration value <10 ng/mL); dashed line AFP high–high group (both baseline and average AFP integration values ≥10 ng/mL)
The incidence rate of HCC in three patient groups, “AFP-low” (a combination of the “low–high” and “low–low” groups),“high–low,” and “high–high”, was estimated using the Kaplan–Meier method and compared using log-rank tests (Fig. 3b). The rate of HCC incidence was significantly higher in the AFP high–high group compared to both the AFP high–low group and patients with low baseline AFP levels (P = 0.009 and 0.001, respectively). There was no significant difference between patients with low baseline AFP levels and the AFP high–low group. The 7-year incidence rate of HCC was 32.3% in the AFP high–high group, compared to only 6.6% in the AFP high–low group, and 8.1% in all patients with low pre-treatment levels.
Discussion
It is well recognized that the most effective strategy for the prevention of HCC development in patients with chronic hepatitis C is likely to be the complete elimination of the HCV infection accompanied by the resultant normalization of liver function [7, 12, 13, 15, 16, 19]. Indeed, we confirmed here that non-SVR is the most significant predictive factor for incidence of HCC in patients receiving interferon therapy for chronic hepatitis C. However, it should be noted that the risk of HCC, even in non-SVR patients, differs between individuals. In the current study, we identified AFP integration value and male gender as independent risk factors for incidence of HCC in non-SVR patients. The incidence of HCC was significantly reduced in individuals with average AFP integration values < 10 ng/mL after interferon therapy, which suggests that the decrease of AFP by interferon therapy lowers the risk of developing HCC. Indeed, even where patients had high baseline AFP levels, incidence of HCC was reduced when the AFP integration value decreased after interferon therapy. Thus, our current findings identify AFP integration value as a useful predictive marker of HCC development in non-SVR patients.
Data from several previous studies suggest that the continuous normalization of alanine aminotransferase (ALT) levels by interferon therapy can reduce the risk of HCC development [36–39]. In addition, one recent study suggested that the ALT integration value is a predictive factor for HCC [35]. In contrast to published data (22), our multivariate analysis did not identify the ALT integration value as a significant predictive factor for HCC incidence, although it was identified as significant by univariate analysis in all 382 patients. Since the previous study did not evaluate AFP levels as a factor for prediction of HCC [35], our results indicate that the AFP integration value is superior to that of ALT as a predictive factor for incidence of HCC. We do not know the reason for this result, but it is speculated that significance of AFP as a marker of hepatic regeneration resulted in the more accurate prediction of hepatocarcinogenesis by integration value of AFP than that of ALT.
As AFP is a diagnostic marker for the existence of HCC, high integration value of AFP in the present study might be a result of HCC development. However, we concluded that the high AFP integration values in patients who developed HCC were not caused by a result of existence of HCC, because of the following two reasons. First, the last AFP values before detection of HCC were not the highest level in the follow-up periods in 19 of 23 patients who developed HCC, suggesting that the AFP was not produced by the developing HCC in these patients. Second, to exclude the influence of the remaining four patients whose last AFP levels were the highest in the follow-up periods, we analyzed the same statistical analysis by using average AFP integration values excluded the last two examinations of AFP before the detection of HCC. The results of the analysis also showed average integration value of AFP as a significant predictive factor for incidence of HCC.
Male gender was also identified as an independent risk factor for HCC in non-SVR patients in this study. Several reports have shown that men are at a higher risk of developing HCC than women [6, 10, 33, 40, 41]. The male gender also appears to be a risk factor for more severe disease and a greater risk of developing cirrhosis in chronic hepatitis C [42]. Although the association of male gender with the risk of HCC is as yet unexplained, hormonal or genetic factors may lead to increased risk for HCC and cirrhosis in men as previously discussed [10].
In conclusion, a decrease in the AFP integration value predicts reduced incidence of HCC in patients with hepatitis C receiving interferon therapy. Further prospective studies with a larger number of patients are required to validate the significance of these findings.
References
Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C, et al. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004–6.
Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006–8.
Hasan F, Jeffers LJ, De Medina M, Reddy KR, Parker T, Schiff ER, Houghton M, Choo QL, Kuo G. Hepatitis C-associated hepatocellular carcinoma. Hepatology. 1990;12:589–91.
Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53.
Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50.
Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, Sato T, Chiba T. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146:649–56.
Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology. 2004;127:S62–71.
Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med. 1999;131:174–81.
Heathcote EJ. Prevention of hepatitis C virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S294–302.
Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48.
Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. International Interferon-alpha Hepatocellular Carcinoma Study Group. Lancet. 1998;351:1535–9.
Camma C, Giunta M, Andreone P, Craxi A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593–602.
Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41.
Fattovich G, Giustina G, Degos F, Diodati G, Tremolada F, Nevens F, Almasio P, Solinas A, Brouwer JT, Thomas H, Realdi G, Corrocher R, Schalm SW. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma, decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1997;27:201–5.
Hayashi K, Kumada T, Nakano S, Takeda I, Kiriyama S, Sone Y, Toyoda H, Shimizu H, Honda T. Incidence of hepatocellular carcinoma in chronic hepatitis C after interferon therapy. Hepatogastroenterology. 2002;49:508–12.
Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Morgan TR. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–9.
Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5.
Okanoue T, Itoh Y, Minami M, Sakamoto S, Yasui K, Sakamoto M, Nishioji K, Murakami Y, Kashima K. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. Viral Hepatitis Therapy Study Group. J Hepatol. 1999;30:653–9.
Izuno K, Fujiyama S, Yamasaki K, Sato M, Sato T. Early detection of hepatocellular carcinoma associated with cirrhosis by combined assay of des-gamma-carboxy prothrombin and alpha-fetoprotein: a prospective study. Hepatogastroenterology. 1995;42:387–93.
Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–5.
Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78:977–85.
Alpert E, Feller ER. Alpha-fetoprotein (AFP) in benign liver disease. Evidence that normal liver regeneration does not induce AFP synthesis. Gastroenterology. 1978;74:856–8.
Bloomer JR, Waldmann TA, McIntire KR, Klatskin G. Alpha-fetoprotein in noneoplastic hepatic disorders. JAMA. 1975;233:38–41.
Ruoslahti E, Seppala M. Normal and increased alpha-fetoprotein in neoplastic and non-neoplastic liver disease. Lancet. 1972;2:278–9.
Sakurai T, Marusawa H, Satomura S, Nabeshima M, Uemoto S, Tanaka K, Chiba T. Lens culinaris agglutinin-A-reactive alpha-fetoprotein as a marker for liver atrophy in fulminant hepatic failure. Hepatol Res. 2003;26:98–105.
Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–32.
Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS, Lee WM, Morgan TR, Ghany MG, Gretch DR. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–41.
Tateyama M, Yatsuhashi H, Taura N, Motoyoshi Y, Nagaoka S, Yanagi K, Abiru S, Yano K, Komori A, Migita K, Nakamura M, Nagahama H, Sasaki Y, Miyakawa Y, Ishibashi H. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46:92–100.
Murashima S, Tanaka M, Haramaki M, Yutani S, Nakashima Y, Harada K, Ide T, Kumashiro R, Sata M. A decrease in AFP level related to administration of interferon in patients with chronic hepatitis C and a high level of AFP. Dig Dis Sci. 2006;51:808–12.
Tamura Y, Yamagiwa S, Aoki Y, Kurita S, Suda T, Ohkoshi S, Nomoto M, Aoyagi Y. Serum alpha-fetoprotein levels during and after interferon therapy and the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci. 2009;54:2530–7.
Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, Hosaka T, Sezaki H, Yatsuji H, Kawamura Y, Kumada H. Prolonged-interferon therapy reduces hepatocarcinogenesis in aged-patients with chronic hepatitis C. J Med Virol. 2007;79:1095–102.
Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, Nakanishi H, Itakura J, Takahashi Y, Kurosaki M, Enomoto N, Izumi N. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518–27.
Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba K, Orito E, Mukaide M, Williams R, Lau JY. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35:201–7.
Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A, Atsumi H, Takagi M, Nakano S, Arakawa T, Fujimori M. Incidence of hepatocellular carcinoma in hepatitis C carriers with normal alanine aminotransferase levels. J Hepatol. 2009;50:729–35.
Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, Hosaka T, Sezaki H, Yatsuji H, Kawamura Y, Kumada H. Interferon-induced prolonged biochemical response reduces hepatocarcinogenesis in hepatitis C virus infection. J Med Virol. 2007;79:1485–90.
Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M, Hino K, Okita K. Risk factors for hepatocellular carcinoma, its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–402.
Kurokawa M, Hiramatsu N, Oze T, Mochizuki K, Yakushijin T, Kurashige N, Inoue Y, Igura T, Imanaka K, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Inui Y, Hijioka T, Yoshihara H, Inoue A, Imai Y, Kato M, Kiso S, Kanto T, Takehara T, Kasahara A, Hayashi N. Effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with chronic hepatitis. Hepatol Res. 2009;39:432–8.
Suzuki K, Ohkoshi S, Yano M, Ichida T, Takimoto M, Naitoh A, Mori S, Hata K, Igarashi K, Hara H, Ohta H, Soga K, Watanabe T, Kamimura T, Aoyagi Y. Sustained biochemical remission after interferon treatment may closely be related to the end of treatment biochemical response and associated with a lower incidence of hepatocarcinogenesis. Liver Int. 2003;23:143–7.
Kurosaki M, Hosokawa T, Matsunaga K, Hirayama I, Tanaka T, Sato M, Yasui Y, Tamaki N, Ueda K, Tsuchiya K, Kuzuya T, Nakanishi H, Itakura J, Takahashi Y, Asahina Y, Enomoto N, Izumi N. Hepatic steatosis in chronic hepatitis C is a significant risk factor for developing hepatocellular carcinoma independent of age, sex, obesity, fibrosis stage and response to interferon therapy. Hepatol Res. 2010;40:870–7.
Takahashi H, Mizuta T, Eguchi Y, Kawaguchi Y, Kuwashiro T, Oeda S, Isoda H, Oza N, Iwane S, Izumi K, Anzai K, Ozaki I, Fujimoto K. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46:790–8.
Forns X, Ampurdanes S, Sanchez-Tapias JM, Guilera M, Sans M, Sanchez-Fueyo A, Quinto L, Joya P, Bruguera M, Rodes J. Long-term follow-up of chronic hepatitis C in patients diagnosed at a tertiary-care center. J Hepatol. 2001;35:265–71.
Acknowledgments
This work was supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osaki, Y., Ueda, Y., Marusawa, H. et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol 47, 444–451 (2012). https://doi.org/10.1007/s00535-011-0505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0505-8