Abstract
Background
Several small, prospective, open studies suggest that leukocytapheresis might be efficient in patients with steroid-dependent ulcerative colitis (UC).
Aim
To evaluate the short- and long-term effectiveness of leukocytapheresis for the management of steroid-dependent UC in clinical practice.
Methods
A Web-based, nationwide database specifically designed to record the efficacy and safety data of leukocytapheresis therapy in UC was available from September 2007 in Spain. Clinical data were collected at treatment baseline, 1 month after the last apheresis session (initial efficacy), and 6 and 12 months thereafter (long-term efficacy). Remission was defined as a Mayo Clinic index ≤2 together with complete steroid withdrawal and response as a decrease of ≥3 from the baseline score.
Results
A total of 142 steroid-dependent UC patients were included in the registry, most of them treated with the Adacolumn™ system. In 69% of patients thiopurine therapy failed to achieve steroid-free clinical remission. Initial clinical remission was obtained in 37% of cases. The initial corticosteroid dose, the number and frequency of apheresis sessions, or the previous failure of thiopurines and/or infliximab did not influence the initial remission rate, but a greater decrease in CRP levels was associated with a higher probability to obtain initial remission. At 6 and 12 months, 41 and 36% of patients were in clinical remission, respectively. Only one serious adverse effect was recorded.
Conclusions
In clinical practice, apheresis allows long-term steroid-free clinical remission in up to one third of steroid-dependent UC patients, even in those with prior failure of thiopurines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic condition of the rectum and colon that usually evolves with recurrent flare-ups of inflammatory activity [1]. Mesalazine and steroids are still the drugs of choice to treat acute flare-ups [2]. Up to one third of those UC patients who require steroid therapy will relapse while tapering the steroid dose or soon after its discontinuation [3, 4], a phenomenon known as steroid-dependence. Long-term use of steroids is inadvisable because of the high rate of side effects and the increased risk of infections. In this clinical setting, only azathioprine, but no other drug, has proven efficacy in RCTs [5], thus becoming the treatment of choice in such patients. Nevertheless, still a considerable proportion of these patients are refractory or intolerant to thiopurines [6, 7]. To avoid colectomy, infliximab therapy may be weighted up, although no specifically designed RCT on steroid-dependent disease has been performed yet [2].
In UC, inflammatory activity is usually associated with an increased number of granulocytes and mononuclear cells in the peripheral blood as well as with mucosal infiltration by inflammatory cells, and has been correlated with the clinical severity and risk of relapse [8, 9]. According to this point of view, removal of activated inflammatory cells from the bloodstream seems an appealing mechanism to reduce mucosal inflammation in UC patients. In fact, several mechanisms of action of leukocytapheresis in inflammatory bowel disease have been recently proposed and reviewed [10], and some open trials and prospective series suggest the usefulness of this therapy in patients with steroid-dependent UC [11–14]. Moreover, the excellent safety profile of leukocytapheresis presents an additional argument for its use in patients that have already been exposed to the severe side effects of corticosteroids. However, although in Spain leukocytapheresis is considered a therapeutic alternative in steroid-dependent UC patients not responding or intolerant to thiopurines [15], strong evidence is still lacking. Monitoring systems allow data collection on the efficacy and safety of emergent medical technologies in real life use [16]. The SiMAC registry (Sistemas de Monitorización de Aféresis en Colitis ulcerosa) was a collaborative project of the Spanish Working Group on IBD (GETECCU) and three regional health technology evaluation agencies from Euskadi, Catalonia and Galicia (Spain). The aims of this monitoring registry were to evaluate the short- and long-term effectiveness and safety of leukocytapheresis therapy to induce steroid-free clinical remission by means of a nationwide registry of clinical practice. In this study, we report the short- and long-term effectiveness of leukocytapheresis in those patients for whom this therapy was specifically prescribed to manage steroid-dependent UC.
Methods
A nationwide, specifically designed registry was available through a website (http://aferesis.geteccu.org) from September 2007 to May 2009, to collect clinical data of all UC patients ever treated with any leukocytapheresis device in Spain on the basis of the GETECCU consensus recommendations [15]. Two hundred one UC patients from 23 centers were included in the registry. Patients were only included if all the required clinical and endoscopic data were available, which meant prospective data collection at the time apheresis therapy was performed. In fact, all the included patients had to have undergone an endoscopic evaluation before starting apheresis and another one within the month following the last ‘induction’ apheresis session. Six patients were excluded because of concomitant use of infliximab. Among the remaining 195 patients, 142 met the criteria for steroid-dependent UC. Clinical and treatment-related data were collected at the time apheresis therapy was started, 1 month after the last induction apheresis session, and 6 and 12 months afterwards. The number, frequency and duration of apheresis sessions, as well as the concomitant use and tapering schedule of steroids, thiopurine introduction or maintenance, and concomitant topical therapy were at physician discretion. C-reactive protein levels were collected whenever available at baseline and 3 weeks after starting apheresis.
Steroid-dependence was defined by the European Crohn’s and Colitis Organization criteria [17]. Clinical remission was defined as a Mayo Clinic score [18] ≤2 together with complete steroid withdrawal, whereas response was defined by a ≥3 point decrease from the baseline value. Endoscopic remission was defined by an endoscopic subscore of ≤1 (erythema, decreased vascular pattern, mild friability). Finally, relapse was defined by a Mayo Clinic score >4 together with the need for a new course of steroids, biologicals, re-apheresis or surgery (oral 5-ASA and topical treatment were permitted) in patients with prior response.
The study was approved by the Institutional Review Board of each participating center. Written informed consent was obtained from all patients.
Statistical analysis
Data are expressed as mean ± standard deviation, median (interquartile range) or frequencies, as required. Wilcoxon’s test was used to analyze CRP variations over time. Chi-square and Mann-Whitney’s U tests were used to evaluate the association of qualitative and quantitative factors, respectively, to response. Survival curves were calculated by the Kaplan-Meier method and compared by the log-rank test. All statistical analyses were performed using SPSS 15.0 package for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients are summarized in Table 1. Of note, most patients (69%) were refractory or intolerant to thiopurines, and infliximab had also been attempted unsuccessfully in 23% of them. Thiopurines were maintained despite prior failure to induce remission in a high proportion of these patients. Thirty-eight additional patients (27%) started thiopurine therapy together with leukocytapheresis, resulting in 68% of the whole cohort using thiopurines concomitantly to apheresis. A majority of patients were concomitantly receiving corticosteroids, as expected. Median corticosteroid dose at the time apheresis was started was 40 mg/day (interquartile range 30–60).
Most patients (95%) were treated with the Adacolumn™ (JIMRO Ltd., Takasaki, Japan), a selective granulocyte and monocyte apheresis system device. Applicability problems were registered in 20% of patients (10% due to difficulties in obtaining peripheral venous accesses). Central lines were required to perform the apheresis sessions in ten patients (7%). In all, a total of 897 apheresis sessions were performed, with a median of 5 sessions (range 1–10) per patient. The procedure was well tolerated, and adverse events were recorded in 18%, most of them mild, with headache being the most common (14%). Only one serious adverse event was noticed (bacterial pneumonia). No deaths occurred.
Initial effectiveness
One month after the last scheduled apheresis session, 97 of the 142 patients (68%) achieved at least a clinical response, with 37% of them (52 patients) already obtaining steroid-free clinical remission at that time. Endoscopic remission was obtained in 41% of patients. Among those patients in clinical remission, 96% also achieved endoscopic remission, with only 23% among those with clinical response.
No differences in clinical remission were found among those patients with previous thiopurine or infliximab failure. The dose of corticosteroids at the time apheresis was neither correlated with initial response nor remission. However, a significant decrease in CRP levels at week 3 was found only among initial responders (from 5.65 to 3 mg/l in responders p = 0.035; from 7.3 to 6 mg/l in non-responders p = 0.166). In the subgroup of patients in whom apheresis was used as a bridge therapy to thiopurines, the response rate was slightly higher (79%), but with similar clinical remission rates (32%).
Long-term effectiveness
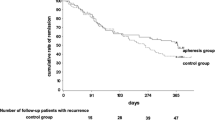
Long-term outcomes are summarized in Fig. 1. Maintenance therapy with monthly apheresis was performed in only 26 patients. Sixty percent of initial responders (41% of the whole cohort) were in clinical remission at 6 months and 54% (36% of the whole cohort) at 12 months. These figures did not vary depending on the use of periodical maintenance apheresis (Fig. 2). Cumulative probability to be in clinical remission at 12 months was significantly higher among patients with initial remission as compared to those with initial response but no remission (p = 0.003).
However, 19 out of the 45 patients (42%) with initial response but not remission achieved clinical remission at 6 months. This ‘late remission’ was not influenced by the concomitant use of corticosteroids or thiopurines, the initial number of apheresis sessions or maintenance therapy with periodical apheresis.
At the end of follow-up, 18 patients (13%) were colectomized because of persistent inflammatory activity in all cases but one (colonic dysplasia while being in remission).
Discussion
In contrast to Japan, leukocytapheresis is not widely used for the management of inflammatory bowel disease in European countries. Despite its excellent safety profile, this technique is not considered a real alternative to steroids for the treatment of acute flare-ups of UC. This can be explained by several factors. First, in Western countries, the more intense time-driven management of acute UC flare-ups that require rapid-acting therapies [19] conflicts with the slow mode of action of leukocytapheresis [20]. Second, the method of steroid use itself, with initial high doses and shorts courses in Europe and low or increasing doses for longer periods in Japan, might influence both efficacy and side effects. Third, the only Western, sham-controlled, RCT of leukacytapheresis in active UC did not demonstrate efficacy in inducing remission [21]. Finally, the lower economic cost of steroid therapy makes the replacement of these drugs by leukocytapheresis as the treatment of choice for acute flare-ups unlikely. However, two recent systematic reviews and meta-analyses confirmed that, although high-quality RCTs in Western populations are still required, leukocytapheresis seems to be of clinical benefit and safer than conventional UC therapies [22, 23]. In fact, the outcomes of some large European case series have already been reported, reflecting the confidence of some gastroenterologists with apheresis [24]. Noteworthily, European efforts have been particularly focused on steroid-dependent UC [11–14, 25], a scenario where apheresis seems to be more efficient compared to severe or refractory flare-ups of the disease [26]. The present study, also focused on steroid-dependent UC, adds valuable information from a large cohort treated in clinical practice. Although it might be handicapped by some potential biases (physician’s own free data collection, lack of a control group, apheresis treatment schedule and concomitant therapies at the physician’s discretion), the strength of this study relies on the large number of patients, the availability of baseline and post-treatment endoscopic features, the hard definition of clinical remission (requiring complete steroid withdrawal) and the systematic use of a monitor system [16].
The efficacy of leukacytapheresis as compared to increasing prednisolone doses in steroid-dependent UC patients was assessed early on by Hanai et al. [27] in a prospective trial including 69 patients. In that study, 11 apheresis sessions were superior to a moderate increase in prednisolone dose (from a median of 12 mg/day to a median of 30 mg/day) in inducing clinical remission at week 12. Our results regarding effectiveness are in agreement with previous data showing that more than one third of patients achieved sustained clinical remission [11–14, 25]. In that setting, apheresis was found to be superior to increasing the steroid dose in inducing clinical remission. Of interest, a decrease in C-reactive protein after 3 weeks of therapy was associated with a higher probability to obtain an initial therapeutic response; this correlates with other studies showing that clinical response could already be predicted after the first three apheresis sessions [28]. There is controversy on the impact of the number and/or the frequency of apheresis sessions on clinical efficacy. Whereas some studies suggest that these issues may be relevant [29, 30], some others point to a little or minimal impact, as in our study [13, 14]. In this sense, the concomitant use of steroids might be a critical issue. Patients who are steroid-naïve seem not to benefit from a greater number of sessions compared to those with partial or no response to steroids [28, 31]. In steroid-dependent disease, the concomitant use of steroids may improve the initial clinical outcome as long as they act as a bridge to the therapeutic effect of apheresis itself. This may explain why, in this clinical setting, reported remission rates have been similar despite the number of apheresis sessions [13, 14]. From the initial apheresis experience in Spain in which a high steroid dose was used at the start of apheresis therapy [12], concomitant steroids are usually maintained and slowly tapered until the last apheresis session. In the present registry, 85 percent of our patients were receiving high doses of steroids at the time apheresis therapy was started, but the tapering schedule was not available. In turn, we cannot rule out that a greater number of sessions might benefit those patients with higher baseline steroid requirements.
The 6-month remission rate was slightly lower than that reported for thiopurines in steroid-dependent UC [5], but it has to be kept in mind that we dealt with a selected refractory population (more than two thirds with prior thiopurine failure) and that our definition of remission included the complete withdrawal of steroids. Among those patients with initial clinical response or remission, monthly re-treatment did not improve the long-term efficacy. On the other hand, when only those patients with prior thiopurine failure where taken into account, maintenance of the immunomodulator therapy showed a non-significant but marked trend toward higher sustained remission at 12 months compared to those patients in whom thiopurines were discontinued (Fig. 3).
As in most apheresis studies, apheresis treatment was well tolerated, with only one serious adverse effect being recorded. Mild headache was the most common reported side effect. It is our thought that this excellent safety profile should be kept in mind when considering rescue therapies in UC patients receiving steroids, thiopurines and/or with co-morbidities in whom the risk of opportunistic infections should be particularly minimized.
In summary, our findings suggest that leukocytapheresis seems to be of clinical benefit in a proportion of patients with steroid-dependent UC even if thiopurines have failed. Once apheresis therapy is started, early assessment of clinical and biological response may be useful; in case of improvement, 5–10 scheduled sessions should be completed and thiopurines maintained even in patients with previous failure. Maintenance therapy with monthly apheresis seems only advisable for those patients intolerant with immunomodulators.
References
Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481–9.
Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, et al. European evidence-based Consensus on the management of ulcerative colitis: current management. J Crohns Colitis. 2008;2:24–62.
Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60.
Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319–30.
Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53.
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30-year review. Gut. 2002;50:485–9.
López-Sanromán A, Bermejo F, Carrera E, García-Plaza A. Efficacy and safety of thiopurine immunomodulators (azathioprine and mercaptopurine) in steroid-dependent ulcerative colitis. Aliment Pharmacol Ther. 2004;20:161–6.
McCarthy DA, Rampton DS. Liu YC. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease Clin Exp Immunol. 1991;6:489–93.
Tibble JA, Sigthorson G, Bridger D. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22.
Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, Winqvist O, et al. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163:50–8.
Cabriada JL, Ibargoyen N, Hernández A, Bernal A, Castiella A. Sustained remission after steroids and leukocytapheresis induced response in steroid-dependent ulcerative colitis: results at 1 year. Dig Liver Dis. 2010;42:432–5.
Domènech E, Hinojosa J, Esteve-Comas M, Gomollón F, Herrera JM, Bastida G, et al. Granulocyteaphaeresis in steroid-dependent inflammatory bowel disease: a prospective, open, pilot study. Aliment Pharmacol Ther. 2004;20:1347–52.
Ricart E, Esteve M, Andreu M, Casellas F, Monfort D, Sans M, et al. Evaluation of 5 versus 10 granulocyteaphaeresis sessions in steroid-dependent ulcerative colitis: a pilot, prospective, multicenter, randomized study. World J Gastroenterol. 2007;13:2193–7.
Dignass AU, Eriksson A, Kilander A, Pukitis A, Rhodes JM, Vavricka S. Clinical trial: five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:1286–95.
Cabriada JL, Domènech E, Gomollón F, González-Carro P, González-Lara V, Hinojosa J, et al. Consensus document on the use of granulocytapheresis in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2006;29:85–92.
Frønsdal KB, Facey K, Klemp M, Norderhaug IN, Mørland B, Røttingen JA. Health technology assessment to optimize health technology utilization: using implementation initiatives and monitoring processes. Int J Technol Asses Health Care. 2010;3:309–16.
Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2:1–23.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9.
Panaccione R, Rutgeerts P, Sandborn WJ, Feagan B, Schreiber S, Ghosh S. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:674–88.
Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568–77.
Sands BE, Sandborn WJ, Feagan B, Löfberg R, Hibi T, Wang T, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–9.
Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci. 2010;55:1421–8.
Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32:1297–306.
Ljung T, Thomsen O, Vatn M, Karlén P, Karlsen LN, Tysk C, et al. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42:221–7.
Kruis W, Dignass A, Steinhagen-Thiessen E, Morgenstern J, Mössner J, Schreiber S, et al. Open label trial of granulocyte apheresis suggests therapeutic efficacy in chronically active steroid refractory ulcerative colitis. World J Gastroenterol. 2005;11:7001–6.
D’Ovidio V, Meo D, Viscido A, Bresci G, Vernia P, Caprilli R. Predictive factors of clinical response in steroid-refractory ulcerative colitis treated with granulocyte-monocyte apheresis. World J Gastroenterol. 2011;17:1831–5.
Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36–44.
Yamamoto T, Umegae S, Kitagawa T, Yasuda Y, Yamada Y, Takahashi D, et al. Granulocyte and monocyte adsorptive apheresis in the treatment of active distal ulcerative colitis: a prospective, pilot study. Aliment Pharmacol Ther. 2004;20:783–92.
Kanke K, Nakano M, Hiraishi H, Terano A. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis. 2004;36:811–7.
Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104:2990–5.
Hanai H, Watanabe F, Takeuchi K, Iida T, Yamada M, Iwaoka Y, et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35.
Acknowledgments
The authors want to dedicate this study to the memory of Antoni Obrador, MD, PhD, who was the president of the Spanish Group for Crohn’s disease and Ulcerative Colitis (GETECCU) at the time this project started. This project was undertaken in the framework of cooperation under the Quality Planning for the Spanish National Health System signed by the Instituto de Salud Carlos III of the Spanish Ministry of Health and the Basque Office for Health Technology Assessment (Osteba).
Conflict of interest
Eugeni Domènech served as speaker, consultant and received research funding; Vicent Hérnandez has received research funding, and José Luis Cabriada and Daniel Ginard served as speakers and consultants to Otsuka Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the GETECCU-SiMAC Group.
Appendix
Appendix
GETECCU-SiMAC group: Aguas M* (Hospital La Fe, Valencia); Arín A (Hospital de Navarra, Pamplona); Barrio J (Hospital Río Hortega, Valladolid); Burrusco MJ (Hospital Virgen del Camino, Pamplona); Cabriada J.L. (Hospital de Galdakao-Usansolo, Galdakao); Clofent J (Hospital de Manises, Valencia); Domènech E* (Hospital Universitari Germans Trias i Pujol, Badalona); Duque J.M. (Hospital San Agustín, Avilés); Estellés J (Hospital Clínico San Carlos, Madrid); Esteve M (Hospital Mútua Terrassa, Terrassa); Fernández F (Hospital Costa del Sol, Marbella-Málaga); Garcia-Planella E (Hospital Santa Creu i Sant Pau, Barcelona); Ginard D (Hospital Son Dureta, Palma de Mallorca); Gómez G (Hospital Doce de Octubre, Madrid); González B (Complexo Hospitalario Universitario de A Coruña, A Coruña); Hernández V (Complexo Hospitalario Universitario, Vigo); Hinojosa J (Hospital de Manises, Valencia); Martínez-López N (Hospital General, Albacete); Merino B (Hospital Gregorio Marañón, Madrid); Muñoz F (Hospital de León, León); Ordás I* (Hospital Clínic, Barcelona); Palau A (Hospital General, Castellón); Pereda A (Hospital Infantil La Fe, Valencia); Roncero O (Hospital La Mancha Centro, Alcázar de San Juan); Saro C (Hospital de Mieres, Gijón); Zabana Y* (Hospital Universitari Germans Trias i Pujol, Badalona).
*Centro de Investigaciones Biomédicas en Red de Enfermedades Hepáticas y Digestivas (Ciberehd).
Rights and permissions
About this article
Cite this article
Cabriada, J.L., Domènech, E., Ibargoyen, N. et al. Leukocytapheresis for steroid-dependent ulcerative colitis in clinical practice: results of a nationwide Spanish registry. J Gastroenterol 47, 359–365 (2012). https://doi.org/10.1007/s00535-011-0499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0499-2