Abstract
Background
We developed a new combined 99mTc-galactosyl human serum albumin (GSA) scintigraphy single-photon emission computed tomography (SPECT)/CT system to evaluate the changes in functional liver volume with portal vein embolization (PVE).
Methods
We performed a prospective analysis of 25 patients treated with right PVE, and evaluated their functional liver volume perioperatively with a 99mTc-GSA scintigraphy SPECT–CT fusion system. The percentage of the non-tumorous remnant liver volume (%LV) and the percentage of functional remnant liver volume (%FLV) were estimated by using the following calculations: (future remnant volume − tumor volume)/(total liver volume − tumor volume) and functional future remnant liver volume/functional total liver volume, respectively.
Results
Before PVE, the correlation was strongly significant between %LV and %FLV of the non-embolized liver, and the data were nearly equal (the regression coefficient was 1.005, P < 0.0001). In contrast, after PVE, there was a significant correlation between %LV and %FLV (P < 0.0001), but the regression coefficient was 1.192. The % LV increased significantly, from 38.1 to 52.0%, and the increment was 13.9% (P < 0.0005). The %FLV was also increased significantly, from 36.6 to 58.0%, and the increment was 21.4% (P < 0.0001). The increment was 7.5% greater for the %FLV compared to that of the %LV (P < 0.001).
Conclusions
The 99mTc-GSA scintigraphy SPECT–CT fusion system can estimate the correct functional liver volume and is useful in comparison with conventional CT volumetry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For patients with advanced hepatic tumors, including hepatocellular carcinoma (HCC), liver metastases, or biliary tract cancer, major hepatic resection is the only possible way to obtain a cure [1–3]. The limitation of the resectable liver area is decided upon an adequate preoperative evaluation of hepatic function and an estimation of the remnant liver volume [4, 5]. The Development of multidetector computed tomography (CT) has made it possible to estimate the correct volumetric measurement of the remnant liver prior to hepatic resection.
Portal vein embolization (PVE) has been developed to expand the indications for hepatic resection and to reduce the risk of postoperative morbidity and mortality [6–8]. PVE may be able to provide not only a larger volume of the future liver remnant, but may also preserve a larger functional volume of the future liver remnant [9–13]. Even in patients who have undergone PVE, the resectability is assessed depending on only the future remnant liver volume when using CT-volumetry, despite the fact that the functions of the embolized liver and non-embolized liver are likely to be very different, reflecting the degree of morphologic atrophy and hypertrophy. Asialoglycoprotein receptors on hepatocytes reflect functional liver cells, and using 99mTc-galactosyl human serum albumin (99mTc-GSA) scintigraphy single-photon emission computed tomography (SPECT), the functional hepatic volume in each lobe of the liver can be measured [9–13]. However, the margin of the left and right liver could not be defined in sufficient detail between the vena cava and the gallbladder, and was identified with reference to the CT scanned metachronously [11, 12].
To obtain correct SPECT and CT fusion images, we developed a new 99mTc-GSA scintigraphy SPECT–CT fusion system. Using the combined SPECT/CT system, SPECT and CT images were obtained on the same bedstead without a position shift, and a more quantitative fusion-image could be obtained because of the various revisions using the attenuation map in the CT. The aim of the present study was to assess the efficacy of this newly developed combined SPECT/CT system for determining the functional liver volume after PVE.
Methods
Patients’ clinical characteristics
Between January - May 2006 and November 2010, a total of 25 patients were treated with right PVE at the Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kumamoto University. Preoperative PVE was mainly indicated for patients with a percentage of non-tumorous remnant liver volume (%LV) smaller than or equal to 35%, with an indocyanine green retention rate at 15 min (ICG R15) of 10% or less, and %LV estimated to be 35–60% with an ICG R15 of 10–20% [8]. The clinical characteristics of the 25 patients are shown in Table 1. The patients consisted of 17 patients with HCCs and 8 patients with other malignancies; 3 intrahepatic cholangiocarcinomas, 2 hilar bile-duct cancers, 2 liver metastases, and 1 gallbladder carcinoma. The diagnosis was made based on a combination of histology, laboratory data, and radiological findings. None of the patients had a serum bilirubin level greater than 2 mg/dl. Three of the 17 patients with HCC underwent selective transarterial chemoembolization at 3 or 4 weeks before the PVE. Our cohort included HCC patients treated with PVE in combination with transarterial chemoembolization (TACE) as multimodal therapy, as there was no indication for liver resection [14]. Six patients were regarded as marginally resectable even after PVE because of their progressive tumor spread, deteriorated liver function, or co-morbidity. One patient refused hepatic resection.
Fifteen patients underwent right-side hepatectomy, and the remaining ten patients did not receive any hepatectomy. The PVE procedure was performed as previously reported [8]. All PVE patients were treated with the percutaneous and transhepatic approach under local anesthesia. We confirmed complete portal vein obstruction by post-portography immediately after PVE and by enhanced CT 1 week after PVE in all patients. All of the patients tolerated the right PVE without any major complications. The Institutional Review Board of the Graduate School of Medical Sciences, Kumamoto University, approved this clinical study. The approval number was 574 and the date was August 1, 2006.
System design
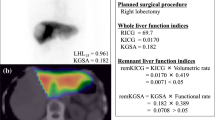
Scintigraphic images were obtained using a SPECT/CT system [15, 16], consisting of a commercially available gantry-free SPECT with dual-head detectors (Skylight; ADAC Laboratories, Milpitas, CA, USA) and an eight-multidetector-row CT scanner (Light-Speed Ultra Instrument; General Electric, Milwaukee, WI, USA) (Fig. 1). The two instruments were juxtaposed such that the CT table carrying the patient could be moved directly into the SPECT scanner before CT scanning. As a result, all the patients were identically positioned for SPECT and CT imaging.
Radionuclide study
All patients were studied with SPECT/CT. After fasting overnight, the patient was placed in a supine position so that the liver and lower part of the heart could be within the field of view of the detectors. 99mTc-DTPA-GSA (Nihon Medi-Physics, Tokyo, Japan) (185 MBq/3 mg) was injected intravenously as a bolus. After confirming that the entire liver was covered by the detectors’ view, SPECT data acquisition was started 20 min after the injection. SPECT data acquisition was performed with a Vertex General Purpose (VXGP) collimator. SPECT data (60 steps of 8 s/step, 360°, 64 × 64 matrix) were obtained. We acquired 64 projections at 8° intervals in the step-and-shoot mode. Between 45 and 60 s was required for each projection; the full study required about 10 min. For SPECT reconstruction, the ordered subsets-expectation maximization (OSEM) algorithm was used (3 iterations, subsets: 8 Butterworth filtering: cutoff = 0.50 cycles/pixel, order = 5.0).
Radiological examination
CT images without contrast administration were obtained at 120 kV, 180 mA, 17.5 mm table feed per rotation, 0.7-s gantry rotation time, 1.25-mm collimation, and 1.25-mm reconstruction. CT images were reconstructed using a standard reconstruction algorithm with a 50-cm field-of-view of the target sites.
Image processing
Reconstructed SPECT and CT images were converted into the DICOM format and then transferred to AZE VirtualPlace LEXUS (AZE, Tokyo, Japan) for fusion imaging (Fig. 2). For image registration, a container (inner diameter 4 mm, length 10 mm) containing an aqueous solution of 99mTc-pertechnetate and contrast medium was used as an external fiducial marker. For the precise registration of both images, external fiducial markers were fixed to the common platform for SPECT and CT imaging; the 2 scans were performed sequentially. Fusion of the SPECT and CT images was performed manually by registration of the external fiducial markers of the 2 images on a workstation. Fusion images were reconstructed in 2 views (axial, and coronal slices). Then three-dimensional (3D) fused images were reconstructed; thus providing the volume-rendering SPECT and CT images.
Measurement of %LV and percentage of the functional remnant liver volume (%FLV)
All of the patients underwent 99mTc-GSA scintigraphy SPECT/CT fusion before and within 3–5 weeks after PVE. The %LV was estimated by using the following calculation: future remnant volume/ (total liver volume − tumor volume). The %FLV) was estimated by using the following calculation: functional future remnant liver volume/functional total liver volume. A correlation analysis was performed for the %LV and %FLV, pre- and post-PVE.
Other clinical parameters
To assess the liver function, ICG R15 and uptake ratio of the liver to the liver plus heart at 15 min (LHL 15), determined by 99mTc-GSA scintigraphy, was measured before and 3–4 weeks after PVE. The hemogram and laboratory data were measured regularly before and after PVE, and after hepatic resection, using standard laboratory methods. Morbidity and mortality related to the PVE procedure and right-side hepatectomy were recorded prospectively.
Statistical analysis
Statistical analyses were performed using the commercial statistical software package IBM SPSS (PASW) Statistics for Windows, version 15.0 (SPSS; Chicago, IL, USA). The values are expressed as means ± SD (range). The Pearson’s correlation product-moment coefficient was used to analyze the correlation between %LV and %FLV before and after PVE. The changes in %LV and %FLV of the left liver before and after PVE were compared using a paired t-test. A P value of less than 0.05 was considered statistically significant.
Results
99mTc-GSA scintigraphy SPECT/CT fusion was performed before and at a mean time of 26 ± 4 days (range 21–30 days) after PVE. The whole liver volumes before and after PVE were similar, at 1255.3 ± 267.4 and 1241.9.8 ± 317.6 cm3, respectively. The embolized right liver volume decreased from 694.2 ± 124.0 cm3 before PVE to 530.0 ± 145.6 cm3 after PVE; in contrast, the left liver volume increased from 442.3 ± 166.2 cm3 before PVE to 586.2 ± 185.9 cm3 after PVE.
Correlation of liver volume and functional liver volume pre- and post-PVE
A correlation between the liver volume and the functional liver volume of the left liver was demonstrated (Fig. 3). Before PVE, the correlation between %LV and %FLV of the left liver was strongly significant, and the regression coefficient was 1.005 (P < 0.0001). In contrast, after PVE, there was a strong significant correlation between %LV and %FLV, but the regression coefficient was 1.192 (P < 0.0001).
Changes in liver volume and functional liver volume pre- and post-PVE
Changes in liver volume and functional liver volume of the left liver were assessed pre- and post-PVE (Fig. 4). The %LV increased significantly, from 38.1 to 52.0%, and the increment was 13.9% (P < 0.0005). The %FLV increased significantly, from 36.6 to 58.0%, and the increment was 21.4% (P < 0.0001). The increment was 7.5% greater for the %FLV compared to that of the %LV (P < 0.001).
In 14 patients with %LV less than 40% pre-PVE, the average %LV increased from 30.3 to 44.1%, and the increment was 13.8%. The average %FLV was also increased, from 28.4 to 48.2%, and the increment was 20.2%. In 15 patients who underwent right hepatectomy, the background liver was histologically analyzed. Six patients were categorized as having severe fibrosis or liver cirrhosis, and the increments of %LV and %FLV in these patients were 17.3% (20.5–57.8%) and 27.0% (37.7–64.7%), respectively.
Changes in liver function and morbidity/mortality in relation to PVE and hepatic resection
The ICG R15 and the LHL 15 data were slightly worse post-PVE, but the differences between pre- and post-PVE were not significant. The ICG R15 increased from 14.7 pre-PVE to 19.3%, and the LHL15 decreased from 0.91 to 0.88. Changes in average values of laboratory data (pre-PVE, post-PVE, and post-resection) were shown for serum albumin (3.8 ± 0.6, 2.9 ± 0.5, and 2.8 ± 0.3g/dl), total bilirubin (1.0 ± 0.5, 1.9 ± 1.2, and 2.0 ± 1.3 mg/dl), and prothrombin activity (93.4 ± 8.7, 78.1 ± 8.6, and 60.8 ± 10.9 %). The post-PVE and post-resection values represented the worst values. There were two patients who met the “50-50 criteria” as a predictive factor of mortality with postoperative hepatic failure [17]. No morbidity was observed in the PVE procedure and there were 3 postoperative complications; two patients with intra-abdominal infection and one with massive ascites. However, no hospital death was encountered in these patients.
Discussion
For liver surgery, the preoperative assessment of the hepatic functional reserve in the predicted remnant liver is important for determining the extent of hepatectomy [4, 5]. Imaging modalities are commonly differentiated as providing either functional or anatomic information. 99mTc-GSA scintigraphy is a well-accepted imaging method for liver function [9–13]. However, SPECT imaging is often insufficient for the precise localization of the remnant liver area when scintigraphy and CT images are viewed side by side. SPECT/CT images provide useful information that can help to differentiate between these two formats. To our knowledge, there have not been any previously reported evaluations of remnant liver function using SPECT/CT fused images. The results of our study showed that the SPECT/CT fused images could lead to an improved ability to evaluate remnant liver function compared to the conventional method measuring the CT volume.
In the present study, PVE demonstrated not only significant hypertrophy in the remnant non-embolized liver, but also functional hypertrophy. Future hepatic functional reserve after hepatic resection used to be estimated according to the volume of the future liver remnant. The basic hypothesis was that liver function per liver volume was equal at each site in the liver. We found that, before PVE, the correlation between %LV and %FLV of the non-embolized liver was strongly significant, and the data were nearly equal (the regression coefficient was 1.005, P < 0.0001). Under normal portal flow and prior to treatment, it is acceptable to estimate the partial liver function corresponding to the partial liver volume. In addition, portal tumor thrombosis, hepatic arterial injury caused by multiple performance of TACE, or obstruction of the bile duct in the future embolized iver can increase the %FLV compared to the %LV. Therefore, it is quite important to have a precise estimation of the remnant liver function before PVE when using the 99mTc-GSA scintigraphy SPECT–CT fusion system.
In contrast to the pre-PVE findings, after PVE, the %LV and %FLV were strongly and significantly correlated, but the %FLV was about 12% larger than the %LV (the regression coefficient was 1.192, P < 0.0001). The increments in %FLV were significantly higher than those in %LV even in patients with pre-PVE %LV less than 40% or histologically severe fibrosis of the liver. The true liver function in the post-PVE status was possibly underestimated by traditional CT volumetry. It has been reported that PVE can result not only in hypertrophy or weight gain in the non-embolized liver, but also increases in heat shock protein, ATP concentration, and DNA synthesis [18]. Heat shock protein can prevent oxidative injury. Increased portal flow in the non-embolized liver after PVE can cause both hypertrophy and hyper-function of the hepatocytes. Mechanisms suspected to cause increases in %FLV and %LV might be different after PVE. In future clinical practice, it can be estimated that 112% of the data of %LV is similar to the %FLV after successful right PVE.
The increments in %LV and %FLV values post-PVE compared with pre-PVE were 14 and 21%, respectively. In HCC patients treated with PVE for the purpose of hemi-hepatectomy, about 10% of the patients still remained unresectable [14]. By taking into consideration the increments of %LV and %FLV, we can now expand the indications for major hepatectomy. Indeed, we can now decide whether a patient is indicated for a hepatic resection according to both the %LV and %FLV. In the present study, of 38 patients treated with right-side hepatectomy, 7 patients were judged to be unresectable based on the %LV, but were considered resectable based on the %FLV, and all 7 patients were able to undergo the operation without any serious complications (unpublished data).
In conclusion, the 99mTc-GSA scintigraphy SPECT–CT fusion system is a new and useful modality for estimating the correct functional volume of the liver compared to conventional CT volumetry.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- PVE:
-
Portal vein embolization
- %LV:
-
Percentage of non-tumorous remnant liver volume
- %FLV:
-
Percentage of functional remnant liver volume
- SPECT:
-
Single-photon emission computed tomography
- 99mTc-GSA scintigraphy:
-
99mTc-galactosyl human serum albumin scintigraphy
- LHL 15:
-
Uptake ratio of the liver to the liver plus heart at 15 min
- ICG R15:
-
Indocyanine green retention rate at 15 min
References
Wei AC, Poon RT, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41.
Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30.
Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–72.
Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–55.
Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–96.
Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunve′n P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7.
Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–75.
Beppu T, Iwatsuki M, Okabe H, Okabe K, Masuda T, Hayashi H, et al. A new approach to percutaneous transhepatic portal embolization using ethanolamine oleate iopamidol. J Gastroenterol. 2010;45:211–7. Epub 2009 Oct 10.
Mitsumori A, Nagaya I, Kimoto S, Akaki S, Togami I, Takeda Y, et al. Preoperative evaluation of hepatic functional reserve following hepatectomy by technetium-99m galactosyl human serum albumin liver scintigraphy and computed tomography. Eur J Nucl Med. 1998;25:1377–82.
Kubo S, Shiomi S, Tanaka H, Shuto T, Takemura S, Mikami S et al. Evaluation of the effect of portal vein embolization on liver function by (99m) Tc-galactosyl human serum albumin scintigraphy. J Surg Res. 2002;107:113–8.
Hirai I, Kimura W, Fuse A, Suto K, Urayama M, et al. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133(5):495–506.
Nanashima A, Yamaguchi H, Shibasaki S, Morino S, Ide N, Takeshita H, et al. Relationship between CT volumetry and functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization before major hepatectomy: a preliminary study. Dig Dis Sci. 2006;51:1190–5.
Kwon AH, Matsui Y, Kaibori M, Ha-Kawa SK. Preoperative regional maximal removal rate of technetium-99m-galactosyl human serum albumin (GSA-Rmax) is useful for judging the safety of hepatic resection. Surgery. 2006;140:379–86.
Okabe K, Beppu T, Masuda T, Hayashi H, Okabe H, Komori H et al. Portal vein embolization can prevent intrahepatic metastases to non-embolized liver. Hepatogastroenterology (in press).
Utsunomiya D, Tomiguchi S, Shiraishi S, Yamada K, Honda T, Kawanaka K, et al. Initial experience with X-ray CT based attenuation correction in myocardial perfusion SPECT imaging using a combined SPECT/CT system. Ann Nucl Med. 2005;19:485–9.
Shiraishi S, Tomiguchi S, Utsunomiya D, Kawanaka K, Awai K, Morishita S, et al. Quantitative analysis and effect of attenuation correction on lymph node staging of non-small cell lung cancer on SPECT and CT. AJR Am J Roentgenol. 2006;186:1450–7.
Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–8.
Tashiro S. Mechanism of liver regeneration after liver resection and portal vein embolization (ligation) is different? J Hepatobiliary Pancreat Surg. 2009;16:292–9.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beppu, T., Hayashi, H., Okabe, H. et al. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT–computed tomography fusion system. J Gastroenterol 46, 938–943 (2011). https://doi.org/10.1007/s00535-011-0406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0406-x