Abstract
Background
Intestinal fatty acid-binding protein (I-FABP) is a low-molecular-mass (15 kDa) cytosolic protein found exclusively in the epithelial cells of the small bowel mucosa. We aimed to evaluate the clinical usefulness of serum I-FABP measurement for the diagnosis of ischemic small bowel disease.
Methods
Patients with a clinical diagnosis of acute abdomen were recruited for this multicenter trial at one university hospital and nine city hospitals over a 13-month period. Serum I-FABP levels were measured in 361 eligible patients by an enzyme-linked immunosorbent assay using a specific monoclonal antibody.
Results
Of the 361 patients, 242 underwent surgery, and small bowel ischemia was diagnosed in 52 patients. The mean serum I-FABP level in the patients with small bowel ischemia was 40.7 ± 117.9 ng/ml, which was significantly higher than that in patients with non-ischemic small bowel disease (5.8 ± 15.6 ng/ml) and those with non-small bowel disease (1.8 ± 1.7 ng/ml). The serum I-FABP cutoff level for the diagnosis of small bowel ischemia was 3.1 ng/ml. Serum I-FABP was more efficient than conventional biochemical markers, in terms of sensitivity and positive and negative predictive values, in the diagnosis of small bowel ischemia. However, its specificity was slightly lower than that of creatinine phosphokinase or lactate dehydrogenase. The positive and negative likelihood ratios of serum I-FABP were 3.01 and 0.29, respectively.
Conclusion
Serum I-FABP measurement is a non-invasive method that is potentially useful for the efficient identification of patients with acute abdomen who are at risk of small bowel ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic diseases of the small bowel, such as occlusion of the superior mesenteric artery and strangulated obstruction of the small bowel, remain life-threatening morbidities [1]. A delay in diagnosis can lead to irreversible full-thickness necrosis of the bowel, which requires massive resection of the small bowel and is associated with a high risk of death. Rapid and accurate diagnosis is essential to improve the prognosis and quality of life of patients with ischemic small bowel disease. Even when the latest high-technology diagnostic devices, such as multidetector computed tomography (CT), are used, these diseases are sometimes overlooked [2, 3]. It remains difficult, even for experienced physicians, to identify patients at risk of bowel necrosis among patients presenting with acute abdomen. Therefore, patients with ischemic small bowel disease tend to be followed up without surgical or radiological intervention before the disease progresses to an advanced stage at which objective signs, e.g., muscular guarding and collapse, are evident.
Organ-specific biomarkers are widely used for the diagnosis of various diseases by blood analysis [alanine aminotransferase for hepatic injury, creatine phosphokinase (CPK) for myocardial infarction, and amylase for pancreatitis], because they are easily measurable and are useful for identifying patients who really need further examination. However, to date, no specific blood marker is available for the detection of injury to the small bowel [4].
Intestinal fatty acid-binding protein (I-FABP) is a cytosolic protein with a molecular mass of approximately 15 kDa [5]. This protein is thought to be important for lipid metabolism in the small bowel, although no confirmative evidence has been unearthed so far [6]. I-FABP is abundant in the mucosa of the small bowel from the duodenum to the distal segment of the ileum and accounts for approximately 2% of cytosolic proteins [5, 7]. These features of I-FABP—a cytosolic protein with a low molecular mass and abundant and specific localization in the intestinal epithelium—make it potentially suitable as a blood marker for the diagnosis of small bowel disease. We have previously shown, using rat experimental models, that the serum I-FABP level increases rapidly in the very early stages of small bowel ischemia [8]. Furthermore, we have demonstrated by analyzing human clinical samples that the serum I-FABP level is elevated in patients with occlusion of the superior mesenteric artery or strangulated obstruction of the bowel [9, 10]. Other investigators have reported elevation of the serum I-FABP level in patients with postoperative necrosis of intestinal grafts [11, 12], necrotizing enterocolitis [13, 14], and ileitis associated with ulcerative colitis [15]. These findings suggest that serum I-FABP holds promise as a biochemical marker for the diagnosis of small bowel disease. However, these studies were retrospective in design and each was conducted at a single institution. Thus, the clinical usefulness of serum I-FABP as a marker of small bowel disease has not yet been determined.
We produced rabbit polyclonal and mouse monoclonal antibodies highly specific to human I-FABP [16] and developed an enzyme-linked immunosorbent assay (ELISA) using these antibodies. These efforts enabled us to measure the I-FABP levels in many clinical serum samples and to perform a multicenter study of serum I-FABP. Here, we report a multicenter validation study that we recently conducted to evaluate the clinical usefulness of serum I-FABP measurement in patients with acute abdominal disease. The primary objective of the study was to evaluate the diagnostic utility of serum I-FABP measurement relative to other biomarkers in patients presenting with acute abdomen. The secondary objective was to evaluate the utility of this marker in the diagnosis of small bowel disease.
Patients and methods
Patients
Patients clinically diagnosed with acute abdomen, i.e., patients whose chief complaint was severe acute abdominal pain and who required judgment regarding the necessity for surgical intervention, were recruited to this multicenter study at Niigata University Hospital and nine other city hospitals in the Niigata district. Between November 2005 and December 2006, 398 patients were enrolled and 37 patients were found to be ineligible for this study: these were 28 patients whose blood samples were not obtained correctly and 9 patients in whom comparative testing data were incomplete. The remaining 361 patients were included in the analysis. This study was approved by the institutional review boards of Niigata University Hospital (BH17-001) and each of the participating institutions, and written informed consent was obtained from all patients.
Procedures
Blood (approximately 3 ml) was collected from each patient within 24 h after the diagnosis of acute abdomen. Serum was immediately separated from each blood sample and stored frozen at −20°C until measurement of I-FABP. Clinical information, including age, sex, and patient’s status, was recorded on registration, and the clinical diagnosis was recorded on the clinical report form immediately after the patient had been discharged. For patients who underwent surgery, additional information, including the diagnosis based on surgical findings, surgical procedures, the presence or absence of bowel obstruction, the presence or absence of small bowel ischemia, and the severity of the ischemia, was recorded. No restriction was imposed concerning the diagnostic imaging technique used, and the responsibility for the surgical indication was assigned to the attending physician. Serum I-FABP levels of all patients were analyzed in a blinded manner at a single laboratory. The results of the I-FABP measurements were not reported to the attending physician and therefore had no effect on the treatment decision-making in any patient.
Serum I-FABP measurement
Serum I-FABP was measured by ELISA, with a rabbit anti-human I-FABP polyclonal antibody serving as the solid phase and a mouse anti-human I-FABP monoclonal antibody as the liquid phase [16]. This ELISA was highly specific for human I-FABP and showed no reaction to the following human FABP species of the same family: heart FABP, liver FABP, adipocyte FABP, brain FABP, or epidermal FABP. This assay allowed quantification of serum I-FABP in the range of 0.1–50 ng/ml. Samples with a value of 50 ng/ml or more were diluted appropriately to determine the I-FABP level. To determine the basal level of human serum I-FABP, we measured serum I-FABP levels in 61 healthy volunteers, using the above-mentioned ELISA technique, before starting the study. The serum I-FABP level in the healthy volunteers was 1.1 ± 0.9 ng/ml, ranging from 0.1 to 5.5 ng/ml (men, 1.2 ± 1.0 ng/ml; women, 1.0 ± 0.5 ng/ml). The features of the ELISA used in this study are described in detail elsewhere [17].
Analysis of diagnostic utility
The serum I-FABP cutoff level for the diagnosis of small bowel ischemia was determined by receiver operating characteristic (ROC) analysis on the basis of the data for 242 patients who underwent abdominal surgery. ROC curves were depicted using JMP software version 6.0.0 (SAS Institute, Cary, NC, USA), varying the potential cutoff levels of serum I-FABP concentration, and the Youden indices [sensitivity − (1 − specificity)] were sequentially calculated. The concentration at which the Youden index showed a maximum value was selected as the cutoff level of I-FABP and was used for the validation of diagnostic utility in the entire group of 361 patients with acute abdomen.
The group of 361 eligible patients was divided into two subgroups: one with small bowel ischemia and one without. We calculated the sensitivity, specificity, and positive and negative predictive values of serum I-FABP for the diagnosis of small bowel ischemia. We also calculated the positive and negative likelihood ratios to evaluate the extent to which the serum I-FABP assay contributed to improving the diagnostic efficacy for small bowel ischemia.
The diagnosis of small bowel ischemia was made on the basis of operative findings. We defined ischemia as the gross disturbance of blood flow in the bowel, regardless of extent and grade. All patients who recovered in response to medical treatment without undergoing surgery were considered to be free of ischemia. One patient who died on the fourth hospital day was clinically diagnosed as having necrotizing enterocolitis. This patient was classified in the small bowel ischemia group in this study, although she did not undergo laparotomy because of her inability to tolerate surgery. No patient in this series was diagnosed by angiography or treated with interventional radiology.
The usefulness of serum I-FABP in diagnosing small bowel disease was also analyzed after dividing the 361 patients into a group with small bowel disease and a group without small bowel disease on the basis of the definite diagnosis recorded on the clinical report form. The definite diagnosis was based on surgical findings in the patients who underwent surgery and, in the patients who did not undergo surgery, the definite diagnosis was based on a comprehensive assessment that included consideration of the patient’s clinical course after the treatment had been undertaken. In this study, patients with generalized peritonitis were classified in the small bowel disease group.
To evaluate the clinical usefulness of serum I-FABP as a biomarker relative to other markers, the following variables were measured simultaneously: (1) inflammation markers [white blood cell count (WBC) and C-reactive protein (CRP)] and (2) enzymes associated with the small bowel [CPK and lactate dehydrogenase (LDH)]. Although these two enzymes are not bowel-specific, they are used empirically as a guide for the diagnosis of bowel necrosis. The normal ranges of these markers used in this study were as follows: WBC, males, 3900–9800/mm3; females, 3500–9100/mm3; CRP, ≤0.3 mg/dl; CPK, males, 38–196 IU/l; females, 30–172 IU/l; LDH, 121–245 IU/l. Each variable was rated as positive if its value exceeded the upper limit of its normal range.
Statistical analysis
The Mann–Whitney U-test was used for intergroup comparisons of quantitative data. Confidence intervals for sensitivity, specificity, positive predictive value, and negative predictive value were calculated using the Wilson score method [18]. Confidence intervals for positive and negative likelihood ratios were calculated by the method described by Simel et al. [19] Statistical significance was set at p < 0.05.
Results
Patients and diagnosis
The clinical characteristics of the 361 patients included in this study are summarized in Table 1. The patients comprised 218 men and 143 women with a mean age of 56.4 years (range 9–97 years). Of the 361 patients, 242 (67.0%) underwent abdominal surgery. The clinical and operative diagnoses of the 361 patients are shown in Table 2. The most frequent disease was bowel obstruction (109 patients, 30.2%), followed by acute appendicitis (95 patients, 26.3%), generalized peritonitis and/or perforation of the gastrointestinal tract (64 patients, 17.7%), incarcerated hernia (30 patients, 8.3%), acute enterocolitis (18 patients, 5.0%), and acute colonic diverticulitis (15 patients, 4.2%). The remaining diseases included acute colitis (4 patients), acute cholecystitis (3 patients), and acute gastritis (2 patients). Only 3 patients with mesenteric infarction were included in this study (0.8%).
The diagnosis of small bowel ischemia was made in 52 of the 361 patients. The diseases of the 52 patients with small bowel ischemia were bowel strangulation (30 patients, 57.7%), incarcerated hernia (15 patients, 28.8%), ischemic enterocolitis (3 patients, 5.8%), mesenteric infarction (3 patients, 5.8%), and low perfusion syndrome due to generalized peritonitis following duodenal perforation (1 patient, 1.9%). In the 309 patients free of ischemia, the most frequent disease was acute appendicitis (95 patients, 30.7%), followed by simple bowel obstruction (79 patients, 25.6%) and peritonitis and/or perforation of the gastrointestinal tract (63 patients, 20.4%).
Distribution of serum I-FABP levels
We show the distribution of serum I-FABP levels for the study group (N = 361) in Fig. 1. The serum I-FABP level of patients in the small bowel ischemia group was 40.7 ± 117.9 ng/ml, which was significantly higher than that of patients in the non-ischemic disease group (3.9 ± 11.6 ng/ml; p < 0.0001). The level in the small bowel ischemia group was also significantly higher than that in the non-ischemic small bowel disease group (5.8 ± 15.6 ng/ml; p < 0.0001). Comparing the two groups without ischemia, the serum I-FABP level in patients with small bowel disease was significantly higher than that in patients with non-small bowel disease (1.8 ± 1.7 ng/ml; p < 0.0001).
Distribution of serum intestinal fatty acid-binding protein (I-FABP) levels in groups with small bowel ischemia, non-ischemic small bowel disease, and non-small bowel disease without ischemia. The distribution of serum I-FABP levels in 61 healthy volunteers is shown in the left column as reference. Mean and SD are indicated by squares and bars, respectively
Cutoff level for diagnosis of small bowel ischemia
To determine the serum I-FABP cutoff level for the diagnosis of small bowel ischemia, ROC analysis was performed on the data of the 242 patients who underwent surgery. Fifty-one patients found to have small bowel ischemia were allocated to the ischemia group, and 191 patients with no ischemia were allocated to the ischemia-free group. The serum I-FABP level and the levels of the other markers (reference markers) were compared between these two groups. The ROC analysis indicated that 3.1 ng/ml was the serum I-FABP cutoff level that allowed valid distinction between the two groups. The area under the ROC curve was greater for serum I-FABP (0.792) than for any of the other markers (Fig. 2).
Patients positive for serum I-FABP
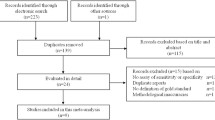
We show a flow diagram of the patient distribution in this study in Fig. 3. There were 52 patients with small bowel ischemia in this study. Of these, 41 (78.8%) were categorized as positive for serum I-FABP (≥3.1 ng/ml). Meanwhile, among the 309 ischemia-free patients, 81 (26.3%) were positive for serum I-FABP.
A total of 122 patients were positive for serum I-FABP, and their profiles are given in Table 3. In the small bowel ischemia group, the most common disease was bowel obstruction (25), followed by incarcerated hernia (10) and ischemic enterocolitis (3). Eighty-one patients showing high serum I-FABP levels were clinically and surgically diagnosed as being free from ischemia. These ischemia-free patients comprised 65 patients with small bowel disease and 16 patients with non-small bowel disease. Of the 65 patients with non-ischemic small bowel disease, 28 required abdominal surgery and 19 required decompression of the gastrointestinal tract using a long tube.
Utility of serum I-FABP in the diagnosis of small bowel ischemia
The utility of serum I-FABP in the diagnosis of small bowel ischemia was analyzed using the data of all 361 eligible patients in the study. Table 4 shows a comparison of the diagnostic utility for small bowel ischemia among serum I-FABP and the other biochemical markers tested. The sensitivity, specificity, positive predictive value, and negative predictive value of serum I-FABP were 78.8, 73.8, 33.6, and 95.4%, respectively. In terms of sensitivity as well as the positive and negative predictive values, serum I-FABP was superior to the other biochemical markers; however, it showed lower specificity (73.8%) than CPK (82.8%) and LDH (77.7%).
The positive and negative likelihood ratios of serum I-FABP were 3.01 and 0.29, respectively, both of which were the best among the ratios observed for the five biomarkers measured in the present study.
Utility of serum I-FABP in the diagnosis of small bowel disease
The 361 patients with acute abdomen were divided into one group with small bowel disease (N = 217) and another group with other diseases (N = 144). The serum I-FABP cutoff level was again set at 3.1 ng/ml. The results of the comparison of serum I-FABP with other markers are shown in Table 5. Except for sensitivity, the indices were higher for serum I-FABP than for the other biochemical markers analyzed.
Discussion
To clarify the diagnostic utility of serum I-FABP, we undertook a multicenter clinical study in patients presenting with acute abdomen. The present study yielded several clinically important findings concerning serum I-FABP measurement. First, this study revealed that the serum I-FABP levels were significantly higher in patients with ischemic small bowel disease than in patients with other diseases associated with acute abdomen and in healthy subjects. Ischemic small bowel disease is a rare occurrence. In our previous study [10], the number of patients with this disease (13 patients) was not large enough for us to conduct a reliable analysis. In the present multicenter study, we analyzed the data of 52 patients with this condition. The mean serum I-FABP level in these 52 patients was 40.7 ng/ml, which was significantly higher than the level in patients with non-ischemic acute abdomen. This result is consistent with the results of our previous study. In the present study, the serum I-FABP level was above the cutoff level in 41 of the 52 patients with small bowel ischemia (78.8%). Thus, the relationship between serum I-FABP and ischemic small bowel disease was demonstrated definitively in the present study.
Second, the present study showed that the serum I-FABP level increased in the presence of not only ischemic disease but also other small bowel diseases. In our previous study that used a polyclonal antibody against an Escherichia coli-expressed recombinant protein, the detection limit with the ELISA was 20 ng/ml or higher. With the ELISA used in the present study, the detection limit was reduced to 0.1 ng/ml because we used a monoclonal antibody to the baculovirus-expressed recombinant protein (which is more akin to human I-FABP in terms of antigenicity) [16]. This improvement in sensitivity allowed us to demonstrate that the serum I-FABP level in healthy persons was 2.0 ng/ml or lower. It also enabled the accurate detection of even a slight elevation in the serum I-FABP level above the normal range. Guthmann et al. [14] reported an elevation of serum I-FABP in children with necrotizing enterocolitis, and Wiercinska-Drapalo et al. [15] reported an elevation in patients with ulcerative colitis. These earlier studies showed that an elevation in blood I-FABP level could be caused not only by ischemia-induced injury of the small bowel mucosa but also by inflammation-induced small bowel injury. The results of the present study indicate that the release of I-FABP into the blood does not ensue specifically in ischemic injury of the small bowel but ensues commonly in small bowel injury caused by diverse types of abdominal diseases, such as acute enterocolitis, simple obstruction of the bowel, and generalized peritonitis. It is also noteworthy that abdominal surgery or decompression with a long tube was required in 47 (72.3%) of our present 65 patients with non-ischemic small bowel disease in whom the serum I-FABP level was above the cutoff level. Recently, Hanssen et al. [20] reported that the blood I-FABP level rose in patients with small bowel injury associated with extracorporeal circulation and that there was a strong correlation between such small bowel injury and the onset of the systemic inflammatory response syndrome. These findings suggest that I-FABP is released into the blood by injury of the small bowel epithelium, regardless of the type of injury, and that the elevation of serum I-FABP in non-ischemic diseases may reflect the extent and seriousness of morbidity.
Third, the present study clearly delineated the usefulness and limitations of serum I-FABP measurement as a means of diagnosing ischemic small bowel disease. In contrast to the good results obtained in terms of sensitivity, the specificity of serum I-FABP was unsatisfactory for the diagnosis of small bowel ischemia. I-FABP failed to exceed the specificity of CPK and LDH, two commonly used biochemical markers related to the small bowel. In our previous animal study [8], CPK was elevated at the stage of obvious ischemia in which necrosis extended to the muscular layer of the small bowel. In contrast, the serum I-FABP level was elevated to as high as 300 ng/ml following 30-min occlusion of the superior mesenteric artery, which caused reversible ischemia that was not associated with morphological changes in the small bowel mucosa. These findings suggest that I-FABP leakage from enterocytes could have been caused by a functional disturbance in the microcirculation that did not lead to macroscopically recognizable necrosis, as the insult was temporary and mild. Such a highly sensitive nature of I-FABP was presumed to have adversely affected the diagnostic specificity for bowel ischemia in the present clinical trial.
In addition, the way of evaluating bowel ischemia should be considered. The grade and extent of ischemia are important factors that could influence the magnitude of the increase in the serum I-FABP level. According to the protocol of the present study, only patients with macroscopically evident signs of ischemia (e.g., a change in the color of the small bowel) were considered to be ischemic. As a result, patients with simple bowel obstruction accompanied by a mild but widely distributed disturbance of circulation due to intestinal dilatation, and patients with disease causing reversible injury of the small bowel mucosa (e.g., generalized peritonitis inducing injury of the small bowel mucosa associated with the systemic inflammatory response syndrome) were classified as being free of small bowel ischemia. Such a binary evaluation system, which is essential for multicenter studies, may have led to the low specificity shown by serum I-FABP when used for the diagnosis of small bowel ischemia.
In the present study, although serum I-FABP showed low specificity for the diagnosis of small bowel ischemia, other indices determining the diagnostic utility of serum I-FABP were better than those of conventionally used biomarkers. We consider it particularly noteworthy that the negative predictive value of serum I-FABP level was as high as 95.4%. In other words, the results of this analysis indicate that if serum I-FABP is measured and revealed to be negative (<3.1 ng/ml), the risk of patients developing small bowel ischemia would be only 4.6%. Current advances in CT are likewise noteworthy. A diagnosis of bowel strangulation can be made with high accuracy by CT [21, 22], i.e., by checking for the lack of enhancement filling in ischemic segments of the small bowel on dynamic CT scans, or by detecting strangulation in reconstructed images by multidetector CT. Therefore, as one of the diagnostic strategies developed for patients with acute abdominal disease, CT is recognized as an essential tool for the diagnosis of patients suspected of being at risk of small bowel ischemia. However, to avoid overlooking a lethal disease, CT tends to be performed more than seems necessary. Meanwhile, there is accumulating evidence suggesting a considerable risk of the occurrence of radiation-induced cancer associated with CT [23]; the estimated risk of fatal cancer induction is reported to be approximately 1 in 1800 [24, 25], and the risk is age-dependent. From the perspective of nationwide health care, this risk should be reduced as much as possible, even if it is acceptably low in each patient. In the present study, the positive likelihood ratio, an indicator for clinical efficacy evaluation, of serum I-FABP in the diagnosis of small bowel ischemia was 3.01. This suggests that we could efficiently refine the identification of patients who are at a high likelihood of small bowel ischemia by measuring the serum I-FABP level. Thus, together with the high negative predictive value of the assay, measuring serum I-FABP levels in patients with acute abdomen to identify those who actually need invasive examinations, including CT, appears reasonable, although whether the serum I-FABP assay can decrease unnecessary CT scans in this setting should be determined in specific clinical trials.
The I-FABP ELISA used in the present study requires approximately 3 h before laboratory results would be available, which is unsatisfactory for clinical use in the setting of the acute abdomen. We are currently developing a simple semiquantitative kit that can yield results within 1 h.
In summary, using a highly sensitive ELISA, we measured serum I-FABP levels in patients with acute abdominal disease. This is the first multicenter study of serum I-FABP measurement in a clinical setting. Serum I-FABP was superior to conventional biochemical markers in the diagnosis of small bowel ischemia, in terms of sensitivity as well as positive and negative predictive values, although its specificity was slightly lower than that of CPK and LDH. The likelihood ratios of serum I-FABP were considered to be useful for indicating patients at risk of small bowel ischemia. We conclude that the serum I-FABP assay is a non-invasive method that is potentially useful for the refined identification of patients with acute abdomen who are at risk of small bowel ischemia.
References
Martin B. Prevention of gastrointestinal complications in the critically ill patients. AACN Adv Crit Care. 2007;18:158–66.
Angelelli G, Scardapane A, Memeo M, Stabile Ianora AA, Rotondo A. Acute bowel ischemia: CT findings. Eur J Radiol. 2004;50:37–47.
Kozuch PL, Brandt LJ. Review article: diagnosis and management of mesenteric ischemia with an emphasis on pharmacotherapy. Aliment Pharmacol Ther. 2005;21:201–15.
Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33:1374–83.
Alpers DH, Strauss AW, Ockner RK, Bass NM, Gordon JI. Cloning of a cDNA encoding rat intestinal fatty acid-binding protein. Proc Natl Acad Sci USA. 1984;81:313–7.
Agellon LB, Toth MJ, Thomson AB. Intracellular lipid binding proteins of the small intestine. Mol Cell Biochem. 2002;239:79–82.
Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–35.
Kanda T, Nakatomi Y, Ishikawa H, Hitomi M, Matsubara Y, Ono T, et al. Intestinal fatty acid-binding protein as a sensitive marker of intestinal ischemia. Dig Dis Sci. 1992;37:1362–7.
Kanda T, Fujii H, Fujita M, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is available for diagnosis of intestinal ischaemia: immunochemical analysis of two patients with ischaemic intestinal diseases. Gut. 1995;36:788–91.
Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–43.
Marks WH, Gollin G. Biochemical detection of small intestinal allograft rejection by elevated circulating levels of serum intestinal fatty acid-binding protein. Surgery. 1993;114:206–10.
Morrisseve PE, Gollin G, Marks WH. Small bowel allograft rejection detected by serum intestinal fatty acid-binding protein is reversible. Transplantation. 1996;61:1451–5.
Gollin G, Marks WH. Elevation of circulating intestinal fatty acid-binding protein in a luminal contents-initiated model of NEC. J Pediatr Surg. 1993;28:367–70.
Guthmann F, Börchers T, Wolfrum C, Wustrack T, Bartholomäus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. 2002;239:227–34.
Wiercinska-Drapalo A, Jaroszewicz J, Siwak E, Pogorzelska J, Prokopowicz D. Intestinal fatty acid-binding protein (I-FABP) as a possible biomarker of ileitis in patients with ulcerative colitis. Regul Pept. 2008;147:25–8.
Kajiura S, Yashiki T, Funaoka H, Ohkaru Y, Nishikura K, Kanda T, et al. Establishment and characterization of monoclonal and polyclonal antibodies against human intestinal fatty acid-binding protein (I-FABP) using synthetic regional peptides and recombinant I-FABP. J Immunoassay Immunochem. 2008;29:19–41.
Funaoka H, Kanda T, Kajiura S, Ohkaru Y, Fujii H. Development of a high-specificity sandwich ELISA system for the quantification of human intestinal fatty acid-binding protein (I-FABP) concentrations. Immunol Invest. 2011;40:1–20.
Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17:2635–50.
Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70.
Hanssen SJ, Derikx JP, Vermeulen Windsant IC, Heijmans JH, Koeppel TA, Schurink GW, et al. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117–25.
Kim JH, Ha HK, Kim JK, Eun HW, Park KB, Kim BS, et al. Usefulness of known computed tomography and clinical criteria for diagnosing strangulation in small-bowel obstructions: analysis of true and false interpretation groups in computed tomography. World J Surg. 2004;28:63–8.
Weisner W, Hauser A, Steinbrich W. Accuracy of multidetector row computed tomography for the diagnosis of acute bowel ischemia in a non-selected study population. Eur Radiol. 2004;14:2347–56.
Berrigton de Gonzalez A, Mahesh M, Bhagavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7.
The 2007 recommendations of the International Commission on Radiological Protection: ICRP publication 103. Ann ICRP. 2007;37:1–332.
Board on Radiation Effects Research (BRER). Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: National Academies Press; 2006.
Acknowledgments
We wish to thank Dr. K. Akazawa, Professor of the Division of Information Science and Biostatistics, Niigata University Graduate School of Medical and Dental Sciences, for helpful advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanda, T., Tsukahara, A., Ueki, K. et al. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: a multicenter, observer-blinded validation study. J Gastroenterol 46, 492–500 (2011). https://doi.org/10.1007/s00535-011-0373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0373-2