Abstract
Background
Virtual touch tissue quantification (VTTQ) based on acoustic radiation force impulse (ARFI) imaging has been developed as a noninvasive bedside method for the assessment of liver stiffness. In this study, we examined the diagnostic performance of ARFI imaging in 103 patients, focusing on the difference in VTTQ values between the right and left liver lobes.
Methods
We evaluated VTTQ values of the right and left lobes in 79 patients with chronic liver disease who underwent histological examination of liver fibrosis and in 24 healthy volunteers. The diagnostic accuracy of VTTQ was compared with several serum markers, including hyaluronic acid, type 4 collagen, and aspartate transaminase to platelet ratio index.
Results
The VTTQ values (meters per second) in the right and left lobes were 1.61 ± 0.51 and 1.90 ± 0.68, respectively, and the difference was statistically significant (P < 0.0001). The VTTQ values in both liver lobes were correlated significantly with histological fibrosis grades (P < 0.001). The standard deviations of the VTTQ values in the right lobe were significantly lower than those in the left lobe (P < 0.001). The area under the receiver-operating characteristic curve for the diagnosis of fibrosis (F ≥ 3) using VTTQ values in both liver lobes was superior to serum markers, especially in the right lobe.
Conclusions
VTTQ is an accurate and reliable tool for the assessment of liver fibrosis. VTTQ of the right lobe was more accurate for diagnosing liver fibrosis than in the left lobe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The management of chronic liver disease depends on the degree of liver fibrosis, and therefore, the assessment of the degree of liver fibrosis is important for choosing a therapeutic strategy and for determining the prognosis [1, 2]. Liver biopsy has been the gold standard method for evaluating the degree of liver fibrosis [3]. However, its invasiveness, potential for life-threatening complications, and sampling errors place a heavy burden on those patients with hepatitis who require follow-up [4–6]. Therefore, noninvasive examination methods for assessing the degree of liver fibrosis, such as serum markers and transient elastography (FibroScan®, Echosens, Paris, France), have been proposed and tried [7–9].
Recently, a method based on acoustic radiation force impulse (ARFI) imaging, Virtual Touch™ tissue quantification (VTTQ), has been introduced to evaluate organ stiffness. The mechanism of VTTQ measurement exploits the phenomena whereby lower displacement magnitudes are induced in cirrhotic liver tissue compared with those induced in noncirrhotic liver tissue, as reported by Nightingale et al. [10]. VTTQ measurements can be performed during observation of a particular liver lesion with an ultrasonic probe, and measurements may be reproducible when compared with transient elastography.
In two pilot studies, it was concluded that liver VTTQ based on ARFI might be useful for the assessment of liver fibrosis in patients with chronic liver disease [11, 12]. In each study, the areas under the receiver-operating characteristic (ROC) curves for F2 were 0.82 and 0.94. However, the number of patients studied was <100, and both study groups were comprised of hepatitis patients, including hepatitis C virus (HCV). Regardless, we observed significant differences in VTTQ values between the right and left lobes of the liver, although this finding has not been reported.
To our knowledge, this is the first report to quantify liver fibrosis stiffness in both the right and left lobe of the liver by VTTQ examination in a study size >100. In this study, we compared the diagnostic accuracy of VTTQ in both the right and left lobes of the liver, using the area under the ROC curves. Furthermore, the diagnostic performance of VTTQ was compared with validated serum fibrosis markers, including the levels of hyaluronic acid and type 4 collagen, and the aspartate transaminase-to-platelet ratio index (APRI).
Patients and methods
Patients
We consecutively enrolled 103 adults, including 24 healthy volunteers (control; n = 24) and 79 patients with or without hepatitis who underwent hepatectomy or living donor liver transplantation (LDLT) and who were measured by VTTQ in the right and the left liver at Kyushu University Hospital. Of these, 73 patients underwent hepatic resection for hepatocellular carcinoma; 46 of these expressed the hepatitis C virus antibody (HCVAb), 7 were positive for hepatitis B virus surface antigen (HBsAg), and 3 were due to alcohol. One patient underwent hepatic resection as a donor for LDLT, and five patients underwent hepatic resection as a recipient. The study protocol conformed to the ethics guidelines of the 1975 Helsinki Declaration and was approved by our institutional review board.
Liver histology and quantification of liver fibrosis
All liver specimens were obtained by surgical resection and were fixed in formalin, embedded in paraffin wax, and stained with hematoxylin and eosin and Masson’s trichrome. The fibrosis staging in all surgical specimens was determined independently by two pathologists who did not know the VTTQ values. In case of discrepancies, histological sections were simultaneously reviewed using a multi-pipe microscope to reach a consensus. Fibrosis staging was scored using the Scheuer classification [16] on a scale of 0–4 as follows: F0, no fibrosis; F1, enlarged, fibrotic portal tracts; F2, periportal or portal-portal septa but intact architecture; F3, fibrosis with architectural distortion but no obvious cirrhosis; F4, probable or definite cirrhosis.
Virtual touch tissue quantification and acoustic radiation force impulse
The VTTQ system was installed on an ACUSON model S2000 ultrasound system (Siemens Medical Solutions Inc., Ultrasound Division, Issaquah, WA). The operators were surgeons trained by Siemens Medical Solutions Inc. The VTTQ system utilizes an acoustic push pulse to generate shear waves, which pass through the liver parenchyma orthogonally to the acoustic push pulse, through a user-placed region of interest. When detection pulses interact with a passing shear wave, they reveal the wave’s location at a specific time, allowing calculation of the shear wave speed. This absolute numerical value is related to the stiffness of the tissue within the region of interest [13–15], and the results are expressed in meters per second (m/s). For each patient, seven successful measurements were performed several days before surgical operations, during which the histological specimens were obtained. A total of 1442 measurements were performed in a total of 103 patients, including 721 measurements in both lobes, respectively. The measurement of VTTQ in the right lobe of the liver was performed by placing the ultrasonic probe on the right intercostal space, and in the left lobe of the liver, measurement was performed by placing the probe under the xiphoid process of the sternum at a depth from 2 to 4 cm. The median value of all measurements and the standard deviation of all right and left VTTQ measurements for each patient were considered for analysis.
Surrogate serum markers
For all patients, blood samples were obtained on the same day that the VTTQ examination was performed and were examined in the same laboratory. The following parameters were determined: levels of hyaluronic acid, type 4 collagen, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and APRI. The APRI was calculated as follows: AST level (per upper limit of normal; 33 U/l) × 100/platelet count (109/l) [16, 17].
Statistical analysis
Differences between quantitative variables for paired samples were analyzed using a nonparametric test (Wilcoxon rank sum test with Bonferroni’s adjustment). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of liver stiffness optimal cutoff values for the diagnosis of liver fibrosis were calculated, as previously published [16, 17]. In addition, the diagnostic value of liver stiffness for predicting significant liver fibrosis (F1–F3) and cirrhosis (F4) was assessed by calculating the areas under the ROC curves. The ROC curve is a plot of sensitivity versus 1-specificity for all possible cutoff values. The most commonly used index of accuracy is the area under the ROC curve, where values close to 1.0 indicate high diagnostic accuracy, and 0.5 indicates a test of no diagnostic value. The optimal liver stiffness cutoff values used for the diagnosis of significant fibrosis and cirrhosis were selected based on the sensitivity, specificity, PPV, and NPV [16, 17, 19]. Statistical analysis of the differences between the areas under the ROC curves was based on the theory of generalized U-statistics [20]. All of the differences were considered statistically significant at P < 0.05.
Results
Patients and liver specimens
Patient characteristics are summarized in Table 1. The mean age of the patients (69 men and 34 women) was 66 ± 12 years. The number of healthy volunteers for the control was 24 and the etiology of hepatitis for 79 patients was classified as follows: hepatitis C (HCV; n = 46), hepatitis B (HBV; n = 7), alcohol (n = 3), and etiology unknown (n = 23). In the patients whose etiology of hepatitis was unknown, HCVAb, HBsAg, and hepatitis B core antibody were negative, and alcoholic hepatitis and nonalcoholic steatohepatitis were not diagnosed clinically. The fibrosis grades of the 79 surgical liver specimens were as follows: F0, n = 12; F1, n = 15; F2, n = 16; F3, n = 15; F4, n = 21, and control, n = 24.
Liver stiffness by virtual touch tissue quantification
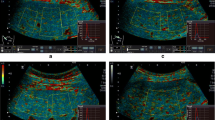
Figure 1 shows box plots of the VTTQ values for each fibrosis stage, for VTTQ values of the right lobe of the liver (Fig. 1a) and left lobe of the liver (Fig. 1b). Liver stiffness values measured by shear wave velocity ranged from 0.74 to 2.88 m/s for the right lobe of the liver and from 0.84 to 3.83 m/s for the left lobe of the liver. The VTTQ values (right/left) in patients with fibrosis grade control (n = 24), F0 (n = 12), F1 (n = 15), F2 (n = 16), F3 (n = 15), and F4 (n = 21) were 1.15/1.41, 1.18/1.41, 1.51/1.85, 1.57/1.77, 1.85/1.88, and 2.10/2.66 m/s, respectively. There were significant correlations between the fibrosis stage and both right and left liver stiffness values (P < 0.001). The VTTQ value (1.61 ± 0.51) of the right lobe of the liver was lower than that of the left lobe of the liver (1.90 ± 0.68), and the difference was statistically significant (P < 0.0001) (Table 2). The standard deviation of VTTQ values in the right lobe (0.23 ± 0.18) was significantly lower than that of the left lobe (0.30 ± 0.17) (P < 0.001). The cutoff values were determined as described above [16, 17]. The optimal cutoff values (right/left) were 1.45/1.84 m/s for F ≥ 1, 1.52/2.16 m/s for F ≥ 2, 1.69/2.24 m/s for F ≥ 3, and 1.79/2.38 m/s for F ≥ 4 (Tables 3, 4). The areas under the ROC curve for the diagnosis of fibrosis types F1, F2, F3, and F4 with the right lobe VTTQ value were 0.81, 0.81, 0.85, and 0.87, respectively (Fig. 2a), and with the left lobe VTTQ value were 0.69, 0.71, 0.76, and 0.86, respectively (Fig. 2b). The area under the ROC curve for the diagnosis of fibrosis (F ≥ 1) with the right lobe VTTQ value was significantly higher than for the left lobe VTTQ value (P < 0.05), and the statistical significance was investigated for the diagnosis of fibrosis (F ≥ 2 and F ≥ 3) (P < 0.05). The area under the ROC curve for each point with the right lobe VTTQ was higher than with the left lobe VTTQ.
Box-and-whisker plot of the VTTQ values for each fibrosis stage. Liver stiffness values measured by shear wave velocity for the right liver lobe (a) and for the left liver lobe (b). The tops and bottoms of the boxes represent the first and third quartiles, respectively. The length of the box thus represents the interquartile range, covering 50% of the values. The line through the middle of each box represents the median. The error bars show the minimum and maximum values (range). Significant correlations were found between the stage of fibrosis and liver stiffness. Statistically significant by the Wilcoxon rank sum test with Bonferroni’s adjustment; *P < 0.01
Diagnostic value of the right VTTQ and the left VTTQ to assess the stage of the liver fibrosis. a The receiver-operating (ROC) curves by the right VTTQ for diagnosing liver fibrosis grade F ≥ 1 (thin black line, area under curve = 0.81), F ≥ 2 (bold black line, area under curve = 0.81), F ≥ 3 (dashed line, area under curve = 0.85) and F ≥ 4 (dotted line, area under curve = 0.87) are shown. b The receiver operating (ROC) curves by the left VTTQ for diagnosing liver fibrosis grade F ≥ 1 (thin black line, area under curve = 0.69), F ≥ 2 (bold black line, area under curve = 0.71), F ≥ 3 (dashed line, area under curve = 0.76) and F ≥ 4 (dotted line, area under curve = 0.86) are shown
Comparison of virtual touch tissue quantification with serum markers for the diagnosis of fibrosis stage ≥3
We compared the area under the ROC curve of the serum markers (hyaluronic acid, type 4 collagen, and APRI) with that of the right lobe VTTQ values, as it was superior to the left VTTQ values for the diagnosis of all fibrosis types. The cutoff values were determined as described before. The optimal cutoff values were 1.69 m/s for the right VTTQ, 2.24 m/s for the left VTTQ, 218.0 ng/ml for hyaluronic acid, 214.0 ng/ml for type 4 collagen, and 0.24 for APRI for the diagnosis of fibrosis stage ≥3 (Table 5). The areas under the ROC curves for the diagnosis of fibrosis (F ≥ 3) according to the right lobe VTTQ, the left lobe VTTQ, hyaluronic acid, type 4 collagen, and APRI cutoff measures were 0.85, 0.76, 0.77, 0.65, and 0.75, respectively (Fig. 3).
Areas under the ROC curves for diagnosing fibrosis grade F ≥ 3 by the VTTQ in the right lobe of the liver, the VTTQ in the left lobe of the liver, and serum levels of hyaluronic acid, type 4 collagen, and APRI. Shown are the ROC curves for diagnosis using the right VTTQ (bold black line, area under curve = 0.85), the left VTTQ (thin black line, area under curve = 0.76), hyaluronic acid level (bold dashed line, area under the curve = 0.77), type 4 collagen level (bold dotted line, area under the curve = 0.65), and APRI (thin dotted line, area under the curve = 0.75)
Discussion
This is the first report to quantify liver fibrosis in both the right and left lobes of the liver in a large population (n = 103) using the VTTQ examination method. The accuracy of right and left lobe VTTQ values for diagnosing liver fibrosis grade F ≥ 3, measured as sensitivity, specificity, PPV, and NPV, was better than serum markers of liver fibrosis, such as the levels of hyaluronic acid, type 4 collagen, and the APRI. This finding is supported by previously reported preliminary data [11, 12].
Many studies have demonstrated that measurement of liver stiffness by transient elastography using FibroScan is a valuable method for assessing liver fibrosis [16–18], and we have previously demonstrated the feasibility of FibroScan for patients with recurrent HCV after LDLT [17]. However, this approach has some limitations when compared with the VTTQ method. First, transient elastography using FibroScan is performed in a blind fashion [17]. Indeed, FibroScan includes a TM screen that shows the ultrasonographic view of the region of interest. However, only large vessels can be distinguished in the image displayed with TM mode in comparison with the B mode standard ultrasonographic image obtained by VTTQ. In most of the previous studies that employed FibroScan, the authors have stated that regions with large vessels were avoided, and a minimal liver parenchyma thickness of 6 cm was sought to minimize the error [16–18]. Nevertheless, these types of acquisition errors can occur because this approach is performed in a blind fashion. This could be the major advantage of VTTQ over transient elastography using FibroScan for the assessment of liver fibrosis. Second, previous reports of elastography in large numbers of the patients have demonstrated that cutoff values between F2 and F3 were close [16–19]. The low predictive value of F2 and F3 could be another limitation of the FibroScan-based elastography. Patients with liver fibrosis of grade F3 or cirrhosis have greater risk of developing hepatocellular carcinoma than those with a liver fibrosis of grade F2. In a study of 2890 patients with hepatitis, Yoshida et al. [21] reported that the annual carcinogenesis rate was correlated with the stage of liver fibrosis. Whereas the annual incidence of hepatocellular carcinoma in patients with severe liver fibrosis of grade F3 was high at 5.3%, the incidence in those with moderate liver fibrosis of grade F2 was low at 1.9%. Furthermore, those patients with a liver fibrosis of grade F3 tended to progress to cirrhosis more easily than those with grade F2 disease. According to a study of 1500 patients with HCV-related chronic hepatitis, Ikeda et al. [22] reported that the progression rate to cirrhosis in patients with liver fibrosis grade F2 was 6.1%, whereas those with liver fibrosis grade F3 was very high at 50.2%, as measured at the end of the 10th year from the start of the observation. They concluded that the fibrotic change was closely correlated with the disease progression rate in patients with viral hepatitis. These findings all suggest that it is critically important to distinguish between liver fibrosis of grade F3 and F2.
Previous studies have not paid much attention to the probe position and the points of measurement when assessing liver fibrosis by VTTQ, as operators are free to measure organ stiffness at any point if they want. Nevertheless, the results of this study suggest that VTTQ measurement with the right lobe is more accurate than with the left lobe when assessing liver fibrosis. The actual values of VTTQ for the right lobe were significantly lower than for the left lobe. The standard deviation of the measured values with the right lobe was significantly lower than with the left lobe. Furthermore, the area under the ROC curve for diagnosing liver fibrosis by the right lobe VTTQ measurement was significantly higher than with the left lobe VTTQ.
Next, we examined the relationship between liver fibrosis stage and VTTQ value in the same lobe of the liver. The lobes of the 79 surgical liver specimens were as follows: right, n = 44 and left, n = 37. The areas under the ROC curve for the diagnosis of fibrosis types F1, F2, F3, and F4 in the right lobe by right VTTQ value (n = 44) were 0.92, 0.83, 0.86, and 0.80, and in the left lobe by left VTTQ value (n = 37) were 0.77, 0.71, 0.78, and 0.84, respectively. The ability to diagnose fibrosis of stage ≥3, which involves sensitivity, specificity, PPV, and NPV, in the right lobe by right VTTQ value were 0.88, 0.81, 0.74, and 0.92, and in the left lobe by left VTTQ value were 0.80, 0.75, 0.87, and 0.68, respectively. Considering these results, it may be concluded that the right VTTQ examination was more accurate for the diagnosis of liver fibrosis than the left VTTQ examination. The cause for this difference remains unknown. The higher standard deviation of the left lobe VTTQ values compared with the right lobe VTTQ values suggests that there may be some difficulties with measurement of VTTQ in the left lobe.
The anatomical features of the left lobe of the liver may have an influence on the measurement of VTTQ. The left lobe is surrounded by the diaphragm, stomach, and aorta, and so may be influenced by respiratory fluctuations, the presence of food in the stomach, and the pulsation of the aorta, respectively. Another factor may be the probe’s position. Few studies of the measurement of liver stiffness using Fibroscan have examined the variability that is possible with different positions of the probe [23]. In almost all of the studies employing FibroScan, the described method was taken from the original description by Sandrin et al. [24], “because liver biopsies are performed on the right lobe of the liver, so were the elasticity measurements. During the acquisition, patients were lying on their backs with their right arms behind their heads. The physician first proceeded to a sonographic examination to localize the best ultrasonic imaging window between the rib bones.” Recently, Ingiliz et al. [23] demonstrated two major points to consider regarding the significance of the probe position and the influence of the skin fold thickness when assessing liver stiffness. First, they showed that the anterior position of the probe should be the first choice for liver stiffness measurement using Fibroscan, as it has a higher applicability without higher variability as compared with the usual liver biopsy position. By contrast, in VTTQ measurements, organ stiffness can be measured at any point that the operators desire because the ultrasonography can be performed during acquisition, and vessels and liver parenchyma thickness are not related to the VTTQ values. Second, in their multivariate analysis, only thoracic skin fold was significantly associated with the variability of the right liver stiffness. Considering this result, the difference in the skin thicknesses between the skin on the right intercostal space and under the xiphoid process of the sternum may be due to the difference in VTTQ between the right and left lobe, and further examination is necessary.
In conclusion, VTTQ examination based on ARFI imaging is an accurate, reliable, reproducible, and noninvasive method with which to assess liver fibrosis of both the right and left lobe of the liver. This approach can be performed with simultaneous observation of individual liver lesions using ultrasonography. The VTTQ measurement of the right lobe may be superior to that of the left lobe for the diagnosis of liver fibrosis, and it may be valuable when assessing patients in whom taking a liver biopsy would be risky, such as those with liver cirrhosis.
Abbreviations
- VTTQ:
-
Virtual Touch™ tissue quantification
- ARFI:
-
Acoustic radiation force impulse
- ROC:
-
Receiver operating characteristic
- ALT:
-
Alanine aminotransferase
- LDLT:
-
Living donor liver transplantation
- APRI:
-
Aspartate transaminase-to-platelet ratio index
- HCVAb:
-
Hepatitis C virus antibody
- HBsAg:
-
Hepatitis B virus surface antigen
- m/s:
-
Meters per second
- AST:
-
Aspartate aminotransferase
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
References
Mazzagferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9.
National Institutes of Health. NIH consensus statement on management of hepatitis C. Hepatology. 2002;36:S3–20.
Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500.
Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–5.
Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8.
Bedossa P, Dargere D, Paradise V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57.
Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75.
Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–92.
Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronans and type III procollagen aminoterminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558–63.
Nightingale KR, Zhai L, Dahl JJ, Frinkley KD, Palmeri ML. Shear wave velocity estimation using acoustic radiation force impulsive excitation in liver in vivo. In: Proceedings of institute of electrical and electronics engineers (IEEE) ultrasonics symposium, 2006. Vancouver: IEEE; 2006. p. 1156–60.
Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604.
Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, et al. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538–45.
Palmeri ML, Frinkley KD, Zhai L, Gottfried M, Bentley RC, Ludwig K, et al. Acoustic radiation force impulse (ARFI) imaging of the gastrointestinal tract. IEEE. 2004;1:23–7.
Dahl JJ, Pinton GF, Palmeri ML, Agrawal V, Nightingale KR, Trahey GE. A parallel tracking method for acoustic radiation force impulse imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:301–12.
Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–68.
Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, et al. Prospective comparison of transient elastography, fibrotest, APRI and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343.
Harada N, Soejima Y, Taketomi A, Yoshizumi T, Ikegami T, Yamashita Y, et al. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation. 2008;85:69–74.
Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Ledinghen V, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48.
Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–8.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the area under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med. 1999;131:174–81.
Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–8.
Ingiliz P, Chhay KP, Munteanu M, Lebray P, Ngo Y, Roulot D, et al. Applicability and variability of liver stiffness measurements according to probe position. World J Gastroenterol. 2009;15:3398–404.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13.
Acknowledgments
The authors wish to thank Siemens Medical Solutions USA, Inc., for the use of the Acuson S2000 device, Mochida Siemens Medical Systems Co., Ltd., in Japan for assisting in the setup of the device, and Ms. Natsumi Yamashita for her valuable advice with the statistical analysis. We have no financial interests linked to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toshima, T., Shirabe, K., Takeishi, K. et al. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol 46, 705–711 (2011). https://doi.org/10.1007/s00535-010-0365-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0365-7