Abstract
Purpose
This randomized, double-blind, controlled study examined whether lafutidine is superior to placebo and non-inferior to famotidine in terms of healing rates as assessed by endoscopy in Japanese patients with mild reflux esophagitis. Safety and improvement in symptoms of heartburn were also assessed.
Methods
Patients with an endoscopic diagnosis of grade A or B reflux esophagitis according to the Los Angeles classification were randomly assigned to receive lafutidine (20 mg/day), famotidine (40 mg/day), or placebo for 8 weeks.
Results
Of the 584 patients enrolled in the study, 447 were diagnosed to have grade A or B reflux esophagitis by the Endoscopic Assessment Committee. Healing rates at 8 weeks were 71.0% (115/162) in the lafutidine group, 61.4% (86/140) in the famotidine group, and 9.7% (14/145) in the placebo group. Lafutidine was thus demonstrated to be superior to placebo and non-inferior to famotidine. As compared with placebo, lafutidine significantly improved symptoms of heartburn.
Conclusions
Lafutidine has a high endoscopic healing rate and improves symptoms of heartburn in patients with mild reflux esophagitis. Lafutidine is considered a promising treatment option for mild reflux esophagitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the prevalence of reflux esophagitis has been increasing in Japan, similar to other developed countries. A recent epidemiological survey performed in Japan estimated that the prevalence of erosive esophagitis according to the Los Angeles classification was 16.7% among patients who underwent initial endoscopy between April through August 2003 [1]. Proton pump inhibitors (PPI) and histamine-2 receptor antagonists (H2RA) are often used for treatment. The effectiveness of PPI and H2RA for reflux esophagitis has been studied internationally, but only rarely in Japan. Many of the studies have revealed that PPI is more effective than H2RA [2, 3]. Meanwhile, H2RA has been used widely in Japan for patients with gastric acid disorders, but adequate evidence supporting the use of H2RA in Japanese patients with reflux esophagitis is thus lacking. Moreover, gastric acid secretion is lower in Japanese than in Westerners [4, 5], and many Japanese patients have mild reflux esophagitis (grade A or B according to the Los Angeles classification) [1, 6, 7]. Adequate evidence using H2RA in such patients is particularly scant.
We previously reported the results of a double-blind, controlled study of lafutidine, a drug with stronger inhibition of gastric acid secretion during the daytime [8] than other H2RA, which is possibly related to modulation of plasma concentrations of calcitonin gene-related peptide (CGRP) and somatostatin [9], in patients with mild reflux esophagitis [10]. Healing rates at 8 weeks (in patients considered eligible by the Endoscopic Assessment Committee) were 67.7% in the lafutidine group, 61.2% in the famotidine group, and 41.2% in the placebo group. However, assessment of the endoscopic photographs differed considerably between the attending physicians and the Endoscopic Assessment Committee. Several tasks remained, including the definition of uniform methods for evaluation of the endoscopic photographs used to assess response.
We thus established clear-cut guidelines for taking and evaluating endoscopic photographs and similarly conducted a randomized, controlled clinical trial to evaluate the efficacy of treatment with lafutidine (whether lafutidine is superior to placebo and non-inferior to famotidine) for 8 weeks in patients with mild reflux esophagitis.
Methods
Study design
The present study was conducted in accordance with Good Clinical Practice guidelines. The study was approved by the institutional review boards of each participating hospital, and written informed consent was obtained from all subjects. This phase III controlled study was conducted from October 2007 until December 2008.
Subjects
Eligibility criteria
Patients who had symptoms of heartburn with a diagnosis of grade A or B reflux esophagitis according to the Los Angeles classification as confirmed by endoscopic examination within 1 week before starting the observation period were eligible for enrollment in the study. The severity and frequency of heartburn were not specified.
Exclusion criteria
Patients with any of the following conditions were excluded: (1) esophageal mucosal breaks that could not be evaluated on endoscopic photographs obtained at the start of treatment to reconfirm disease status; (2) gastric or duodenal ulcers (excluding ulcer scars); (3) the concurrent presence of Barrett’s esophagus; (4) a history of a poor response to H2RA or PPI given in the recommended dose for 8 weeks; (5) less than 24 weeks had elapsed since the completion of Helicobacter pylori eradication therapy with a successful outcome; (6) a history of upper gastrointestinal resection; and (7) other conditions considered by the attending physician to potentially affect the assessment of efficacy and safety.
Study methods
To eliminate placebo effects and carry-over effects from previous treatment, placebo was administered for 1 week in a single-blind fashion during the observation period. The study was terminated at the end of the observation period in patients in whom heartburn disappeared.
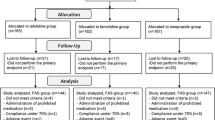
The duration of treatment was 8 weeks. Patients were randomly assigned to receive lafutidine (10 mg, twice daily), famotidine (20 mg, twice daily), or placebo. Endoscopic examinations were repeated at the completion of treatment to evaluate the healing rates in each treatment group. During the study, the number of episodes of heartburn was evaluated by reviewing the patients’ diaries. In addition to endoscopy, physical examinations and laboratory tests were performed to confirm the eligibility and safety of the patients (Fig. 1).
Serum antibody profiles were analyzed to confirm the presence or absence of H. pylori infection. In patients in whom H. pylori had been successfully eradicated, urea breath tests were conducted, irrespective of the results of serum antibody analysis, and the results were adopted. The presence or absence of hiatal hernia and of gastric mucosal atrophy assessed according to the Kimura–Takemoto classification [11] were also determined on endoscopic examination at study entry.
Study drugs and randomization
Using the add-in macro program of Microsoft Excel, the person responsible for treatment assignment prepared a computer-generated list of random numbers with the use of a permuted-block procedure. The randomization code for treatment allocation was preassigned by using an add-in macro random-number generator and was saved.
Lafutidine 10-mg tablets were used as the study drug. Famotidine 20-mg tablets and lafutidine and famotidine placebo tablets identical in appearance to the actual drugs were used as control drugs. Because lafutidine tablets and famotidine tablets differ in size, the double-dummy method was used, and patients received 2 tablets per time, twice daily. The assignment ratio was lafutidine/famotidine/placebo = 1:1:1.
Concomitant drugs and therapy
During the study, the use of other H2RA, PPI, gastric mucosal protective agents, prokinetic agents, antacids, and antispasmodic agents was prohibited. Basically, treatment with any other drug was not started during the study period. However, aluminum hydroxide gel plus magnesium hydroxide suspension (Mulfa® Suspension, Ono Pharmaceutical Co., Ltd., Osaka, Japan) could be used as required to control heartburn.
Drug compliance and laboratory testing
Compliance was considered good if patients took 75% or more of their assigned drugs. Laboratory tests were performed every 4 weeks.
Evaluation methods
Method for taking endoscopic photographs
Written descriptions of the evaluation criteria used by the Endoscopic Assessment Committee and the Los Angeles classification were provided to the investigators at the participating hospitals. To ensure that uniform methods were used for evaluation, the investigators were requested to take endoscopic photographs after the gastrointestinal tract had been adequately washed and insufflated, with the squamocolumnar junction fully distended.
Healing rates as assessed by endoscopy
The primary endpoint was the healing rate as assessed by endoscopy at the end of the study. The Los Angeles classification was used to endoscopically evaluate reflux esophagitis. Cases were considered as healed if their grade changed to grade O by the endoscopy at the end of study. The evaluation made by the Endoscopic Assessment Committee was designated as “the committee’s evaluation”, and that made by the attending physicians was designated as “the physicians’ evaluation”. The committee’s evaluation was used for primary analyses, and the physicians’ evaluation was used for secondary analyses. To derive the committee’s evaluation, each committee member individually evaluated endoscopic findings. If all members agreed on the grade of esophagitis, that grade was used as the committee’s evaluation. If even 1 member considered esophagitis to be a different grade, the committee reviewed the case until a unanimous consensus was reached. That grade was then used as the committee’s evaluation. The Endoscopic Assessment Committee consisted of 4 experienced endoscopists. All endoscopic findings were evaluated in a blinded fashion.
Symptoms of heartburn
The patients’ diaries were used to assess the severity and the number of episodes of heartburn. The severity of heartburn was graded with the use of a visual analogue scale.
Safety
The frequency and rate of side effects were assessed for patients who took either of the study drugs at least one time (i.e., the safety analysis set).
Statistical analysis
Analysis of healing rates as assessed by endoscopy
-
1.
To examine whether lafutidine is superior to placebo, a chi-squared test (continuity correction) was performed, with a one-sided significance level of 2.5%.
-
2.
Only if lafutidine was proved to be superior to placebo in analysis 1, the non-inferiority of lafutidine to famotidine was examined with the Dunnett–Gent test, with a non-inferiority margin of 10% and a one-sided significance level of 2.5%.
Analysis of symptoms of heartburn
Patients considered eligible by the Endoscopic Assessment Committee were included in the analysis. The number of episodes and the severity of heartburn were assessed during the observation period and after 2, 4, 6, and 8 weeks of treatment, and the mean values were calculated. The percent changes in these variables as compared with the baseline values during the observation period were calculated for each evaluation period. The treatment groups were then compared with the use of Student’s t test or Wilcoxon rank sum test for each evaluation period, with a two-sided significance level of 5% and no correction for multiplicity.
Analysis of safety
The frequency and rate of side effects were compared between the treatment groups. Side effects occurring at a frequency of 1.0% or higher were defined as main side effects.
Results
Study population
Figure 2 shows the breakdown of the subjects included in efficacy analyses. Of the 584 patients who gave written informed consent to participate in the study and entered to the observation period, 83 discontinued the study during the observation period and 501 were assigned to treatment (safety analysis set). The main reasons for discontinuation of the study were the inability to reconfirm mucosal breaks on endoscopic photographs taken at the start of treatment (26 patients) and the withdrawal of informed consent (22 patients). Of the 501 patients assigned to receive treatment, 28 were excluded from efficacy analyses, mainly because endoscopy was not performed (14 patients) or the allowable range of endoscopic examinations deviated from the study protocol (5 patients).
A total of 473 patients were included in efficacy analyses (physicians’ evaluation): 165 patients in the lafutidine group, 150 in the famotidine group, and 158 in the placebo group. Of the 473 eligible patients according to the physicians’ evaluation, 26 (5.5%) were considered ineligible by the Endoscopic Assessment Committee on the basis of the endoscopic photographs taken at enrollment. Therefore, 447 patients were included in the committee’s evaluation: 162 in the lafutidine group, 140 in the famotidine group, and 145 in the placebo group.
Demographic information
Table 1 shows the demographic characteristics of the 447 patients considered eligible by the Endoscopic Assessment Committee. The presence or absence of hiatal hernia and of smoking differed among the 3 groups (P = 0.03 and P = 0.02, respectively).
Table 2 shows the demographic characteristics of the 473 patients included in the physicians’ evaluation. In these patients, only the distribution of the presence or absence of smoking differed among the 3 groups (P = 0.04).
Healing rates as assessed by endoscopy
Analysis according to the committee’s evaluation
Healing rates were 71.0% (115/162) in the lafutidine group, 61.4% (86/140) in the famotidine group, and 9.7% (14/145) in the placebo group. Statistical analysis showed that lafutidine was superior to placebo and non-inferior to famotidine (Fig. 3). According to disease grade, the healing rates for grade A esophagitis were 79.2% (80/101) in the lafutidine group, 68.3% (56/82) in the famotidine group, and 11.3% (8/71) in the placebo group. The healing rates for grade B esophagitis were 57.4% (35/61), 51.7% (30/58), and 8.1% (6/74), respectively (Fig. 4).
According to H. pylori infection, the healing rates for H. pylori positive patients were 77.8% (21/27) in the lafutidine group, 62.1% (18/29) in the famotidine group, 8.0% (2/25) in the placebo group. The healing rates for H. pylori negative patients were 70.1% (94/134), 61.3% (68/111), 10.1% (12/119), respectively. Statistical analysis has not showed a significant difference between positive and negative patients in each group by Fisher’s exact test. The healing rates for patients with gastric mucosal atrophy (i.e., present) were 70.8% (51/72) in the lafutidine group, 66.7% (48/72) in the famotidine group, 13.6% (9/66) in the placebo group. The healing rates for patients without gastric mucosal atrophy (i.e., absent) were 71.1% (64/90), 55.9% (38/68), 6.3% (5/79), respectively. Statistical analysis has not showed a significant difference between patients with and without each item in each group by Fisher’s exact test.
Analysis according to the physicians’ evaluation
Healing rates were 69.1% (114/165) in the lafutidine group, 64.0% (96/150) in the famotidine group, and 14.6% (23/158) in the placebo group, demonstrating that lafutidine was superior to placebo and non-inferior to famotidine (Fig. 5). According to disease grade, the healing rates for grade A esophagitis were 77.0% (77/100) in the lafutidine group, 71.4% (60/84) in the famotidine group, and 17.5% (14/80) in the placebo group. The healing rates for grade B esophagitis were 56.9% (37/65), 54.5% (36/66), and 11.5% (9/78), respectively (Fig. 6).
According to H. pylori infection, the healing rates for H. pylori positive patients were 78.6% (22/28) in the lafutidine group, 70.0% (21/30) in the famotidine group, 25.9% (7/27) in the placebo group. The healing rates for H. pylori negative patients were 67.6% (92/136), 62.5% (75/120), 12.3% (16/130), respectively. Statistical analysis has not showed a significant difference between positive and negative patients in each group by Fisher’s exact test. The healing rates for patients with gastric mucosal atrophy were 67.6% (50/74) in the lafutidine group, 68.4% (52/76) in the famotidine group, 19.2% (14/73) in the placebo group. The healing rates for patients without gastric mucosal atrophy were 70.3% (64/91), 59.5% (44/74), 10.6% (9/85), respectively. Statistical analysis has not showed a significant difference between patients with and without each item in each group by Fisher’s exact test.
Symptoms of heartburn
Percent change in the number of episodes of heartburn
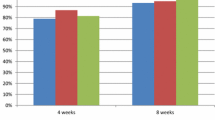
The percent changes in the number of episodes of heartburn according to the committee’s evaluation are shown in Table 3. Both lafutidine and famotidine significantly decreased the numbers of episodes of heartburn after 2, 4, 6, and 8 weeks of treatment, as compared with placebo.
Severity of heartburn
The percent changes in the severity of heartburn during the daytime according to the Committee’s evaluation are shown in Table 4. Both lafutidine and famotidine significantly decreased the severity of heartburn after 2, 4, 6, and 8 weeks of treatment, as compared with placebo.
Side effects
Side effects occurred in 8.9% (15/169) of the lafutidine group, 5.5% (9/163) of the famotidine group, and 4.7% (8/169) of the placebo group. The main side effects in the lafutidine group were elevated alanine aminotransferase levels, elevated γ-glutamyl transpeptitase levels, decreased white-cell counts, and increased serum uric acid concentrations. The main side effects in the famotidine group were diarrhea and increased total bilirubin levels. No serious side effects developed in either group.
Discussion
Many studies have reported that PPIs are effective for the management of reflux esophagitis [2, 3]. The “Guidelines for the Evaluation and Treatment of Gastroesophageal Reflux Disease” issued by the Japanese Society of Gastroenterology in 2009 recommend PPIs as the treatment of choice for gastroesophageal reflux disease.
Recently, ischemic cerebrovascular disease has been increasing in Japan. Low-dose aspirin is widely used for the treatment, and clopidogrel is often used concurrently. But it has been reported that low-dose aspirin increases the risk of gastrointestinal bleeding [12] and induces gastroesophageal injury itself [13, 14]. Furthermore, PPIs have been reported to decrease the effectiveness of clopidogrel when used concurrently [15]. The usage of antiplatelet agents is expected to increase in the future owing to the rapid growth of the elderly population. Such elderly patients may require H2RA for the treatment of reflux esophagitis. H2RA can be used as a treatment option in patients unable to receive PPIs. Our results demonstrated that lafutidine is superior to placebo and non-inferior to famotidine according to both the committee’s evaluation and the physicians’ evaluation.
According to the committee’s evaluation, the healing rates were 71.0% (115/162) in the lafutidine group and 61.4% (86/140) in the famotidine group. The healing rates for grade A esophagitis were 79.2% (80/101) in the lafutidine group and 68.3% (56/82) in the famotidine group, and those for grade B esophagitis were 57.4% (35/61) and 51.7% (30/58), respectively. Better healing rates were thus obtained in patients with grade A mild reflux esophagitis. Milder reflux esophagitis is strongly associated with gastroesophageal reflux during the daytime [16]. Lafutidine, which effectively suppresses gastric acid secretion during the daytime, apparently produced a higher healing rate.
Both lafutidine and famotidine significantly improved symptoms of heartburn, as compared with placebo. Although it has been unclear why improvement of heartburn of placebo became much higher at 8 weeks than 2 weeks, it was reported that the percentage of days without heartburn of placebo at 1 week was 33%, which led to 55% at 8 weeks, in the study of famotidine and placebo performed with nonerosive reflux disease patients [17]. Furthermore, placebo effect could be higher because we set the baseline symptoms of heartburn only once or more in the observation period in our study. H2RAs have been reported to inhibit gastric acid secretion more promptly than PPIs and are therefore expected to rapidly improve symptoms of heartburn [18–21].
In our study, we clearly defined the evaluation criteria and the procedures for taking endoscopic photographs. We therefore believe that the study group comprised patients with clear-cut endoscopic findings of reflux esophagitis, i.e., distinct mucosal breaks strongly associated with acid regurgitation. Consequently, the proportion of patients considered ineligible by the Endoscopic Assessment Committee was only 5.5% (26/473), considerably lower than that in our previous study [10] (27.1%, 91/336).
The healing rate in the placebo group according to the committee’s evaluation was 9.7% (14/145), which was much lower than that in our previous study (41.2%, 21/51). In studies performed outside of Japan, the healing rate in patients with reflux esophagitis who received 8 weeks of treatment with placebo has widely ranged from 12 to 36%, depending on the investigator [22–26]. Because these studies used the Hetzel–Dent classification, a direct comparison with the results of our study, which used the Los Angeles classification, is not feasible. However, some studies using the Los Angeles classification have reported that the results differed among operators and evaluators [27, 28], suggesting that healing rates may differ even when the Los Angeles classification is used. The low healing rate in the placebo group in our study may be related to the fact that all subjects had to have mild reflux esophagitis that was associated with distinct mucosal breaks, which were strongly attributed to acid reflux as a result of definition of evaluation criteria and procedure for taking endoscopic photographs. These strict criteria, may have contributed to the low healing rate in the placebo group. Differences in patient characteristics may also be related to the low healing rate in the placebo group. At study entry, the prevalence of grade B esophagitis according to the Los Angeles classification was 51.0% (74/145) in the placebo group, which was higher than that in our previous study (37.3%, 19/51). This higher prevalence of grade B (i.e., severer) esophagitis in the present study may have also led to the lower healing rate. However, at study entry the proportions of patients with grade A or grade B esophagitis according to the Los Angeles classification were generally similar in the lafutidine and famotidine groups.
In clinical practice, patients should carefully undergo endoscopic examinations. The optimal treatment regimen should be selected after comprehensively evaluating patients’ conditions, risk factors, and symptoms.
Our results showed that lafutidine improved healing rates as assessed by endoscopy as well as symptoms of heartburn in patients with mild reflux esophagitis. These promising results suggest that lafutidine is an important treatment option for mild reflux esophagitis.
References
Ohara S, Kouzu T, Kawano T, Kusano M. Nationwide epidemiological survey regarding hertburn and reflux esophagitis in Japanese (in Japanese with English abstract). Nippon shokakibyo Gakkai Zasshi (JSSG). 2005;102:1010–24.
Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane database Syst Rev. 2007;18:CD003244.
Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–100.
Haruma K, Kamada T, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, et al. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277–83.
Feldman M, Cryer B, Mcarthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–52.
Fujimoto K, Iwakiri R, Okamoto K, Oda K, Tanaka A, Tsunada S, et al. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol. 2003;38:3–6.
Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518–34.
Koike T, Ohara S, Sekine H, Kawamura M, Abe Y, Inomata Y, et al. Effect of Helicobacter pylori status on intragastric pH during administration of lafutidine or famotidine. Hepatogastroenterology. 2007;54:1280–4.
Shimatani T, Inoue M, Kuroiwa T, Xu J, Nakamura M, Tazuma S, et al. Lafutidine, a newly developed antiulcer drug, elevates postprandial intragastric pH and increases plasma calcitonin gene-related peptide and somatostatin concentrations in humans: comparisons with famotidine. Dig Dis Sci. 2006;51:114–20.
Ohara S, Haruma K, Kinoshita Y, Kusano M. Efficacy evaluation of Lafutidine for mild reflux esophagitis in Japanese patients (in Japanese with English abstract). Nippon Shokakibyo Gakkai Zasshi (JSSG). 2010;107:588–97.
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97.
Sakamoto C, Sugano K, Ota S, Sakaki N, Takahashi S, Yoshida Y, et al. Case-control study on the association of upper gastrointestinal bleeding and nonsteroidal anti-inflamatory drugs in Japan. Eur J Clin Pharmacol. 2006;62:765–72.
Tutuian R. Clinical lead outpatient services and gastrointestinal function laboratory. Adverse effects of drugs on the esophagus. Best Pract Res Clin Gastroenterol. 2010;24:91–7.
Ruszniewski P, Soufflet C, Barthelemy P. Nonsteroidal anti-inflammatory drug use as a risk factor for gastro-oesophageal reflux disease: an observational study. Aliment Pharmacol Ther. 2008;28:1134–9.
Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu JV, Henry DA, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713–8.
Adachi K, Fujishiro H, Katsube T, Yuki M, Ono M, Kawamura A, et al. Predominant nocturnal acid reflux in patients with Los Angeles grade C and D reflux esophagitis. J Gastroenterol Hepatol. 2001;16:1191–6.
Hongo M, Kinoshita Y, Haruma K. A randomized, double-blind, placebo-controlled clinical study of the histamine H2-receptor antagonist famotidine in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:448–56.
Adachi K, Komazawa Y, Mihara T, Azumi T, Fujisawa T, Katsube T, et al. Comparative study of the speed of acid-suppressing effects of oral administration of cimetidine and famotidine. J Gastroenterol Hepatol. 2005;20:1012–5.
Yamagishi H, Koike T, Ohara S, Horii T, Kikuchi R, Kobayashi S, et al. Stronger inhibition of gastric acid secretion by lafutidine, a novel H2 receptor antagonist, than by the proton pump inhibitor lansoprazole. World J Gastroenterol. 2008;14:2406–10.
Inamori M, Togawa J, Iwasaki T, Ozawa Y, Kikuchi T, Muramatsu K, et al. Early effects of lafutidine or rabeprazole on intragastric acidity: which drug is more suitable for on-demand use? J Gastroenterol. 2005;40:453–8.
Suzuki T, Yamaguchi T, Odaka T, Kobayashi M, Seza A, Kouzu T. Four-day continuous gastric pH monitoring following anti-acid secretory drug administration: cross-over test to assess the early effects. Aliment Pharmacol Ther. 2008;27:66–71.
Simon TJ, Berenson MM, Berlin RG, Snapinn S, Cagliola A. Randomized, placebo-controlled comparison of famotidine 20 mg b.d. or 40 mg b.d. in patients with erosive oesophagitis. Aliment Pharmacol Ther. 1994;8:71–9.
Silver MT, Murdock RH, Morrill BB, Sue SO. Ranitidine 300 mg twice daily and 150 mg four-times daily are effective in healing erosive oesophagitis. Aliment Pharmacol Ther. 1996;10:373–80.
Cloud ML, Enas N, Humphries TJ, Bassion FS. Rabeprazole in the treatment of acid peptic diseases: results of three placebo-controlled dose-response clinical trials in duodenal ulcer, gastric ulcer and gastroesopageal reflux disease (GERD). The Rabeprazole Study Group. Digest Dis Sci. 1998;43:993–1000.
Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo-controlled, randomized clinical trial. Panatoprazole US GERD Study Group. Am J Gastroenterol. 2000;95:3071–80.
Sontag SJ, Hirschowitz BI, Holt S, Robinson MG, Behar J, Berenson MM, et al. Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: the US multicenter study. Gastroenterology. 1992;102:109–18.
Kusano M, Ino K, Yamada T, Kawamura O, Toki M, Ohwada T, et al. Interobserver and intraobserver variation in endoscopic assessment of GERD using the “Los Angeles” classification. Gastrointest Endosc. 1999;49:700–4.
Nasseri-Moghaddam S, Razjouyan H, Nouraei M, Alimohammadi M, Mamarabadi M, Vahedi H, et al. Inter- and intra-observer variability of the Los Angeles classification: a reassessment. Arch Iranian Med. 2007;10:48–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohara, S., Haruma, K., Kinoshita, Y. et al. A double-blind, controlled study comparing lafutidine with placebo and famotidine in Japanese patients with mild reflux esophagitis. J Gastroenterol 45, 1219–1227 (2010). https://doi.org/10.1007/s00535-010-0283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0283-8