Abstract
Background
Alpha-fetoprotein (AFP), lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), des-γ-carboxy prothrombin (DCP), and Golgi protein-73 (GP73) have been used or proposed as tumor markers for hepatocellular carcinoma (HCC).
Methods
They were measured in 96 patients undergoing hepatectomy for HCC to investigate their treatment response and association with variables linked with tumor invasiveness and/or prognosis. Values at 1 month post-surgery in the 77 patients without recurrence within 6 postoperative months were adopted as those after surgery.
Results
GP73 levels did not change after hepatectomy, but levels of other markers decreased and areas under receiver operating characteristic curves (95% CI) were: 0.64 (0.56–0.72), 0.63 (0.55–0.71), 0.79 (0.73–0.86), and 0.63 (0.55–0.71) for AFP, AFP-L3, DCP, and combination of AFP and AFP-L3, respectively. Cutoff points giving specificities of 96.1% (sensitivities at these points) were: 124 ng/mL (28.1%), 10% (21.9%), and 60 mAU/mL (52.1%), for AFP, AFP-L3, and DCP, respectively. The combination of AFP and AFP-L3 provided a sensitivity of 26.0% at a specificity of 96.1%. The increased DCP value was, or tended to be, associated with a larger tumor, vascular invasion, intrahepatic metastases, and a lower grade of tumor cell differentiation. Although similar associations were found between AFP and vascular invasion as well as a lower grade of tumor cell differentiation, no such relationship was found with AFP-L3.
Conclusions
DCP is a more effective tumor marker than AFP and AFP-L3. AFP-L3 showed comparable accuracy to AFP but no benefit was found in their combination. GP73 did not play a significant role in this context. Indices of tumor invasiveness were most closely associated with DCP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of death from cancer worldwide and its incidence has been increasing in countries where the incidence of HCV infection is increasing [1]. Serum alpha-fetoprotein (AFP) has been used as a de facto standard biological tumor marker of HCC since the 1970s. However, AFP can be elevated in patients with chronic hepatitis and/or cirrhosis in the absence of HCC, leading to an unreliable role of AFP in surveillance [2].
To date, several other tumor markers have been investigated as complements for AFP. Plasma des-γ-carboxy prothrombin (DCP), also known as protein induced by vitamin K deficiency or antagonist-II (PIVKA-II), was first reported in 1984 [3]; and it has been widely used for two decades in Japan, especially since 1997 when DCP could be measured with a tenfold higher sensitivity [4]. The lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3) has also been proposed as a marker for HCC, and has been commonly used in Japan since the 1990s. AFP-L3 is a fucosylated variant of AFP and the percentage of AFP-L3 over total AFP levels is used as an index of HCC [5]. AFP-L3 is reportedly more specific to HCC than AFP, representing its malignant potential [6]. In the meantime, Golgi protein-73 (GP73) has recently been shown to have a superior diagnostic ability to AFP [7, 8].
In addition to their use as diagnostic tools for surveillance, biological tumor markers play several important roles in the following aspects: monitoring treatment response, as indices of specific clinicopathological variables that provide prognostic information, and detecting disease relapse after curative treatment [9]. However, these aspects have never before been investigated in a comprehensive manner.
In the present study, we investigated the roles of AFP, DCP, AFP-L3, and GP73 as HCC tumor markers, paying particular attention to these unaddressed issues in patients with HCC undergoing liver resection.
Materials and methods
Patients
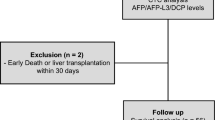
Between June 2007 and August 2008, 116 consecutive patients who were scheduled to undergo liver resection for suspected HCC were enrolled and followed prospectively at the Hepato-Biliary-Pancreatic Surgery Division of Tokyo University Hospital, Tokyo, Japan (Fig. 1). The study protocol was approved by the institutional ethics board and written informed consent was obtained from each subject before treatment. Preoperative diagnosis of HCC was made by using abdominal ultrasonography and dynamic computed tomography (CT) scanning. Other imaging modalities were added if necessary. The indication of liver resection was determined according to previously described criteria [10].
Curative resection was defined as the removal of all recognizable tumors with a clear margin. HCC diagnosis was finally confirmed by pathological examination of the resected specimens in all cases.
From these 116 patients, 96 were finally included. Twenty patients were excluded for the following reasons: three by the presence of extrahepatic metastases found intraoperatively, two due to non-curative liver resection, eight in whom pathological examination revealed non-HCC tumors (intrahepatic cholangiocarcinoma in five, inflammatory pseudotumors in two, focal nodular hyperplasia in one), three due to the incomplete pathological examination as a result of total necrosis of the tumor, and four by the prescription of warfarin, a DCP-inducing agent (Fig. 1). Patients’ background characteristics and tumor characteristics are presented in Tables 1 and 2, respectively.
Follow-up after hepatectomy
Monthly follow-up was conducted by assessment of tumor markers (AFP, DCP, and AFP-L3) and ultrasound. Dynamic CT scan was conducted at 3 and 6 months post-surgery. We defined recurrence as the appearance of new lesions with radiological features typical of HCC, as confirmed by at least two imaging methods [11].
Tumor marker measurement
Blood samples for tumor markers were taken both 7 days prior to and 1 month after liver resection. Serum AFP levels were measured by an immunometric assay (ST AIA-PACK AFP, Tosoh, Tokyo, Japan). Serum AFP-L3 levels were measured by lectin-affinity electrophoresis coupled with antibody-affinity blotting (LBA AFP-L3, Wako Pure Chemical Industries, Osaka, Japan), and were expressed by the ratio of AFP-L3 to total AFP (%) [5, 12]. AFP-L3 levels were not detected when AFP concentrations were <10 ng/mL, thus AFP-L3 values were defined as 0% in this range [13]. Plasma DCP levels were measured by the two-step enzyme immunoassay (Picolumi PIVKA-II, Eizai, Tokyo, Japan) [4]. Serum GP73 autoantigen and GP73 autoantibody levels were measured by prototype enzyme-linked immunosorbent assays (Quanta Lite™ GP73 Antigen ELISA and Quanta Lite™ GP73 Antibody ELISA, INOVA Diagnostics Inc., San Diego, USA). Assay results were assessed spectrophotometrically and expressed as optical densities (OD).
Relationship between tumor markers
In the measurement of multiple tumor markers, marker values should ideally be independent to each other. With this in mind, we assessed the relationship between each tumor marker before liver resection.
Ability of tumor markers to assess therapeutic response
The marker values of 96 patients before liver resection were defined as those with HCC. Of these, 77 patients remained free of recurrence 6 months after liver resection. Marker values of these 77 patients 1 month post-surgery were defined as values at complete tumor remission. To assess the ability of tumor markers to reflect the therapeutic response after curative resection, we constructed receiver operating characteristic (ROC) curves, and calculated the areas under ROC curves (AUROCs). The sensitivity/specificity at several cutoff points which were conventionally used and of specific interest in the present study were also calculated.
AFP-L3 is always measured simultaneously with AFP and its significance depends on that of AFP [6]. Similarly, AFP-L3 is thought to play a role in patients with intermediately elevated AFP values, because of its high specificity [6, 14]. With this in mind, the significance of the AFP-L3 measurement in addition to AFP was investigated through the ROC curve constructed by combining the two assays. In the combination assays, three different cutoff ranges were set as follows: in the low (AFP value <20 ng/mL) and the high (AFP value ≥400 ng/mL) cutoff ranges, cutoff points were varied according to AFP values; whereas in the intermediate cutoff range (20 ng/mL ≤ AFP value < 400 ng/mL), cutoff points were varied according to AFP-L3 values where AFP values <20 ng/mL were always classified into marker negative while AFP values ≥400 ng/mL were classified into marker positive. For example, when the AFP-L3 value of 15% was adopted as the cutoff value in the intermediate cutoff range, a patient with AFP of 800 ng/mL was classified as marker positive even when the AFP-L3 value was 5%. Here, the transition point of AFP at 400 ng/mL was adopted according to the EASL 2000 criteria [15].
Association of tumor marker values with clinicopathological variables representative of tumor invasiveness and prognosis
We assessed the association of respective marker values with clinicopathological variables that have been reported as being representative of tumor invasiveness and/or poor prognosis. Variables were assessed pathologically on the resected specimens (Table 2). Vascular invasion was defined as the presence of portal vein invasion, venous invasion, or biliary invasion. Multiple primary tumor nodules and intrahepatic metastases were differentiated by using the guidelines proposed by the Liver Cancer Study Group of Japan [16].
Alteration of marker positive/negative status through hepatectomy and postoperative marker positive status as an early indicator of tumor recurrence
We examined the alteration in the marker positive/negative status through treatment in a patient-by-patient manner. Then, we assessed the association between marker positive/negative status and tumor recurrence during the early postoperative phase, recurrence within 6 months of liver resection.
Statistical analysis
Marker values were expressed as medians with inter-quartile ranges. Correlations between marker values were analyzed by Spearman’s rank correlation (r S). AUROCs for markers were compared by Wilcoxon’s rank sum test [17]. Associations between marker values and clinicopathological variables were analyzed by Wilcoxon’s rank sum test or by the Kruskal–Wallis test, as appropriate. P values <0.05 were accepted as statistically significant. All statistical analyses were performed using the GraphPad Prism® computer software, version 5 (GraphPad Software Inc., San Diego, CA, USA).
Results
Relationship between tumor markers
The values of AFP and AFP-L3 showed a close association (r S = 0.83), and those of GP73 autoantigen and GP73 autoantibody were moderately related (r S = 0.48). No significant correlation was found in any of the other combinations of tumor marker values (Table 3).
Ability of tumor markers to assess therapeutic response
Tumor marker values of 96 patients before liver resection and those of 77 patients 1 month post-surgery in whom no recurrence was detected until 6 months post-surgery are depicted in Fig. 2.
Tumor marker values before hepatectomy (N = 96) and 1 month after hepatectomy (N = 77). Values after hepatectomy exclude the 19 patients whose tumor recurred within six postoperative months. a AFP, b AFP-L3, c DCP, d GP73 autoantigen, e GP73 autoantibody. Marker distributions are expressed by scatter dot plots and box and whiskers. Top and bottom of boxes are first and third quartiles, respectively. Length of box represents inter-quartile range within which 50% values were located. Line through middle of each box represents median. Error bars show minimum and maximum values (range). Figures above box and whiskers represent medians (inter-quartile ranges). Dashed lines represent cutoff values most frequently used in clinical settings and as follows: AFP, 20 ng/mL; AFP-L3, 10%; DCP, 40 mAU/mL
Since GP73 did not appear to be a tumor marker representing tumor status, the following analyses exclude GP73 autoantigen and GP73 autoantibody results. The overall abilities of these tumor markers and the combination of AFP and AFP-L3 to reflect the therapeutic response after curative resection are depicted in Fig. 3.
Receiver operating characteristic (ROC) curves comparing tumor markers and corresponding area under ROCs (AUROCs) (95% CI). a AFP versus AFP-L3. P = 0.73. b AFP versus DCP. P < 0.005. c AFP or AFP-L3 versus combination of AFP and AFL-L3. P = 0.16 (AFP vs. combination of AFP and AFP-L3) and P = 0.27 (AFP-L3 vs. combination of AFP and AFP-L3), respectively
Sensitivity/specificity at various cutoff points for these tumor markers is demonstrated in Table 4. The sensitivity of DCP was higher than those of other markers at a specificity of 96.1%, while sensitivities of other markers were of a similar extent. At a specificity of 97.4%, sensitivities of AFP-L3 and the combination of AFP and AFP-L3 were 16.7 and 26.0%, respectively (P = 0.12).
Association of tumor marker values with clinicopathological variables representative of tumor invasiveness and prognosis
Correlations between AFP, AFP-L3, and DCP values and clinicopathological variables are shown in Table 2. Increased DCP value was associated with the indices representing tumor growth and invasiveness such as tumor size, presence of vascular invasion, and lower grade of tumor cell differentiation. Although similar, albeit moderate, tendency was observed in the relationship between AFP value and these variables, no apparent association was found between AFP-L3 value and these indices.
Alteration of marker positive/negative status through hepatectomy and postoperative marker positive status as an early indicator of tumor recurrence
In this analysis, the cutoff points for various marker values were set at those which gave the equivalent specificities taking into account conventionally used values [18]. They were 200 ng/mL for AFP, 10% for AFP-L3, and 60 mAU/mL for DCP (Table 4). Specificities at these points were 97.4% for AFP, 96.1% for AFP-L3, 96.1% for DCP, and 96.1% for the combination of AFP and AFP-L3. Analysis was conducted in patients with positive preoperative marker status. The rates of patients who still had marker positive status 1 month post-surgery were as follows: 6/21 (28.6%) for AFP, 6/21 (28.6%) for AFP-L3, 4/50 (8.0%) for DCP, and 8/25 (32.0%) for the combination of AFP and AFP-L3. In these patients, imaging-proven recurrences within six postoperative months were detected with the following incidences: 4/6 (66.7%), 4/6 (66.7%), 2/4 (50.0%), and 5/8 (62.5%) for AFP, AFP-L3, DCP, and the combination of AFP and AFP-L3, respectively. Whereas, recurrence rates within six postoperative months for those patients whose preoperative positive marker status turned negative 1 month post-surgery were as follows: 4/15 (26.7%), 2/15 (13.3%), 11/46 (23.9%), and 3/17 (17.7%) for AFP, AFP-L3, DCP, and the combination of AFP and AFP-L3, respectively. Therefore, the risk of developing early postoperative recurrence in patients whose marker status remained positive 1 month post-surgery was higher than in patients whose marker values changed to negative status. This was expressed as the risk ratio (95% CI) in Table 5: 2.5 (0.9–6.9), 5.0 (1.2–20.5), 2.1 (0.4–25.3), 3.5 (1.1–11.3) for AFP, AFP-L3, DCP, and the combination of AFP and AFP-L3, respectively.
Discussion
AFP, AFP-L3, and DCP have been widely used in Japan for screening and monitoring treatment response and/or relapse [19, 20]. Ideally, levels of tumor markers should fall to within a normal range after effective treatment and rise before the tumor relapse is detected by imaging studies. This aspect is especially important in the case of transcatheter arterial embolization and chemotherapy, because radiological findings do not necessarily reflect the degree of biological remission achieved by necrosis or fibrosis [21].
HCC biomarkers have also been reported to substitute as markers of specific clinicopathological variables representing the malignant potential of the tumor. In cases of non-surgical therapy, markers could therefore provide prognostic data when pathological information are unobtainable; and in cases of liver resection and transplantation, they may do so prior to the treatment [22]. In the present investigation, we evaluated these aspects of AFP, AFP-L3, and DCP as well as GP73.
GP73 antigen expression is barely detectable in normal subjects, but is strongly upregulated in the hepatocytes of patients with acute hepatitis, cirrhosis, and during the progression of chronic liver disease. GP73 was also a promising serum marker for HCC in preliminary studies [7, 8]. In the present study, however, neither GP73 autoantibody nor GP73 autoantigen levels appear to reflect the tumor status (Fig. 2). Other studies also reported the insufficiency of serum GP73 as an HCC-specific marker, although they confirmed that it may be a marker for chronic liver diseases or hepatitis C virus-related HCC [23, 24]. It should be noted that GP73 was increased in patients with liver disease, in particular, with the advancement of disease; HCC usually develops in the later stages of hepatitis C virus infection; and all previous studies suggesting the significance of GP73 as an HCC tumor marker were cross-sectional. Hence, it is more appropriate to consider that high levels of GP73 in patients with HCC reflect the fact that HCC develops at the advanced stage of chronic liver diseases.
To date, several studies have assessed the diagnostic accuracies of AFP-L3 and/or DCP in comparison with that of AFP through ROC curves. Two studies examined the significance of AFP-L3 and two of them reported that it was comparable to that of AFP [25, 26]. Five studies evaluated DCP [27–31], and four of these reported the superiority of DCP over AFP [27, 28, 30, 31]. Two studies examined the accuracies of AFP-L3 and DCP simultaneously in comparison with AFP [14, 32]. The former appeared to rank diagnostic accuracies in the decreasing order of DCP, AFP, and AFP-L3 [14], although no statistical comparison was done. The latter reported that AFP, which showed similar accuracy to DCP, was superior to AFP-L3 [32].
The present analyses revealed that DCP was superior to AFP while AFP-L3 was comparable to AFP (Fig. 3; Table 4). This finding agrees with the general conclusion from previous studies. We must bear in mind, however, that although AFP is inferior to DCP as a single tumor marker, they are independent markers and thus thought to be complementary to each other (Table 3). Furthermore, we sought the utility of additional measurements of AFP-L3 given known AFP values based on the considerations described earlier. It is of interest that the diagnostic accuracy of the combination of AFP with AFP-L3 was equivalent to AFP alone but superior to AFP-L3 alone (Fig. 3; Table 4). These results argue that the additional measurement of AFP-L3 to AFP is not mandatory, and that to the contrary, AFP-L3 data should always be interpreted in reference to AFP.
A high specificity has been reported as a feature of AFP-L3 [6]. The apparent discrepancy of the present results from reported characteristics can not be explained straightforwardly. One possible explanation may be related to the limitation of this study, that is, the present cohort comprised those undergoing hepatectomy. The majority of patients had one or two HCC nodules and the tumor diameter was relatively larger than that of the non-surgical cohort. The significance of AFP-L3 may be more marked in patients with multiple and/or small HCC nodules. Recently, a newly developed micro-total analysis system (μ-TAS) was reported not only to have higher analytical sensitivity than current methods in the determination of AFP-L3 but also to have the ability to measure AFP-L3 at a lower total AFP concentration [33]. Further studies by use of the μ-TAS system are expected to answer the question unaddressed in the present study.
Although the association of tumor markers with clinicopathological variables has been evaluated in many studies, the majority of these works only assessed associations with variables of interest and/or exclusively for AFP, AFP-L3, or DCP. Variables were also usually assessed by radiological findings or in specimens obtained by biopsy. In the present study, we investigated these associations in a comprehensive manner using pathological findings of resected specimens (Table 2). An elevated DCP value was broadly associated with variables representing tumor invasiveness and/or poor prognosis (Table 2). A similar, moderate association was confirmed for AFP but not AFP-L3, although a similar trend was observed. This agrees in part with previous studies, which showed that respective tumor markers stood for specific pathological indices, for example, AFP for poor degree of tumor cell differentiation [34, 35], AFP-L3 for poor degree of tumor cell differentiation and presence of vascular invasion [36–38], and DCP for the presence of vascular invasion and/or intrahepatic metastasis [34, 39, 40].
One of the unique features of the present study is that we were able to follow the alterations in tumor marker values before and after the hepatectomy in a patient-by-patient manner. At cutoff points giving fairly high specificities (96.1–97.4%), almost all patients who had been negative for respective tumor markers before the hepatectomy also remained marker negative after the operation. By contrast, a considerable proportion of patients who had been marker positive did not attain marker negative status even after the curative liver resection (Table 5). Later follow-up revealed that this had been the unidentified sign of recurrence. This prediction ability appeared to be most prominent in AFP-L3 compared with AFP or DCP. This observation is in line with previous reports that suggested the significance of AFP-L3 lies in the early recognition of HCC in the follow-up of patients with cirrhosis [6].
In conclusion, DCP was shown to be a better tumor marker than AFP and AFP-L3 in monitoring the treatment response in patients with HCC, but AFP was a useful complementary marker to DCP. The accuracy of AFP-L3 was comparable to that of AFP, but no benefit was found in the additional measurement of AFP-L3 to AFP. Conversely, AFP-L3 values should be interpreted in reference of those of AFP. GP73 was not an HCC marker under the present clinical conditions. Correlation of tumor marker values with clinicopathological variables representing the malignant potential of HCC and/or poor prognosis was strongest for DCP, followed by AFP and AFP-L3 in this order. The significance of the AFP-L3 measurement may lie in the early recognition of tumor recurrence after treatment.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AFP-L3:
-
Lens culinaris agglutinin-reactive fraction of AFP
- AUROC:
-
Areas under ROC curve
- CI:
-
Confidence interval
- DCP:
-
Des-γ-carboxy prothrombin
- GP73:
-
Golgi protein-73
- HCC:
-
Hepatocellular carcinoma
- ROC curve:
-
Receiver operating characteristic curve
References
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34.
Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Eng J Med. 1984;310:1427–31.
Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94:650–4.
Aoyagi Y, Isemura M, Suzuki Y, Sekine C, Soga K, Ozaki T, et al. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet. 1985;2:1353–4.
Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoprotein in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–23.
Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–12.
Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Med Oncol. 2009;27:339–45.
Yuen MF, Lai CL. Serological markers of liver cancer. Best Pract Res Clin Gastroenterol. 2005;19:91–9.
Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rate of hepatocellular carcinoma: a randomized trial. Lancet. 2000;356:802–7.
Shimizu K, Taniichi T, Satomura S, Matsuura S, Taga H, Taketa K. Establishment of assay kits for the determination of microheterogeneities of alpha-fetoprotein using lectin-affinity electrophoresis. Clin Chim Acta. 1993;214:3–12.
Toyoda H, Kumada T, Kaneoka Y, Osaki Y, Kimura T, Arimoto A, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol. 2008;49:223–32.
Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–8.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Liver Cancer Study Group In Japan. The general rules for the clinical and pathological study of primary liver cancer. 5th ed. Tokyo: Kanehara; 2008.
Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98.
Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30.
Tateishi R, Shiina S, Yoshida H, Teratani T, Obi S, Yamashiki N, et al. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology. 2006;44:1518–27.
Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51.
Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–59.
Hasegawa K, Imamura H, Ijichi M, Matsuyama Y, Sano K, Sugawara Y, et al. Inclusion of tumor markers improves the correlation of the Milan criteria with vascular invasion and tumor cell differentiation in patients with hepatocellular carcinoma undergoing liver resection (#JGSU-D-07–00462). J Gastrointest Surg. 2008;12:858–66.
Gu Y, Chen W, Zhao Y, Chen L, Peng T. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Ann Clin Biochem. 2009;46:38–43.
Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its values as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49:1602–9.
Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, et al. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394–402.
Sterling RK, Jeffers L, Gordon F, Sherman M, Venook AP, Reddy KR, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol. 2007;102:2196–205.
Marrero JA, Su GL, Wei W, Sherman M, Venook AP, Reddy KR, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–21.
Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, et al. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6115–9.
Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, et al. Sensitivity and specificity of des-gammma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma varies according to tumor size. Am J Gastroenterol. 2006;101:2038–43.
Kim do Y, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72:52–7.
Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, et al. Significance of alpha-fetoprotein and des-γ-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:1795–804.
Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8.
Kagebayashi C, Yamaguchi I, Akinaga A, et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal Biochem. 2009;388:306–11.
Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S, et al. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032–8.
Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, et al. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311–6.
Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Yamamoto M, et al. Clinicopathologic features of patients with hepatocellular carcinoma seropositive for alpha-fetoprotein-L3 and seronegative for des-gamma-carboxy prothrombin in comparison with those seropositive for des-gamma-carboxy prothrombin alone. J Gastroenterol Hepatol. 2002;17:772–8.
Tada T, Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25:848–53.
Miyaaki H, Nakashima O, Kurogi M, Eguchi K, Kojiro M. Lens cullinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol. 2007;42:962–8.
Inoue S, Nakao A, Harada A, Nonami T, Takagi H. Clinical significance of abnormal prothrombin (DCP) in relation to postoperative survival and prognosis in patients with hepatocellular carcinoma. Am J Gastroenterol. 1994;89:2222–6.
Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73:2464–71.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (B) (20390352) and Mitsui Life Social Welfare Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, K., Imamura, H., Matsuyama, Y. et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol 45, 1272–1282 (2010). https://doi.org/10.1007/s00535-010-0278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0278-5