Abstract
Background
Nonalcoholic steatohepatitis (NASH) is a feature of metabolic syndrome. Advanced glycation end-products (AGEs) are formed by the Maillard reaction, which contributes to aging and to certain pathological complications of diabetes. A recent study has suggested that glyceraldehyde-derived AGEs (Glycer-AGEs) are elevated in the sera of patients with NASH. Furthermore, immunohistochemistry of Glycer-AGEs showed intense staining in the livers of patients with NASH. The present study aimed to examine the effect of intracellular Glycer-AGEs on hepatocellular carcinoma (Hep3B) cells.
Methods
Cell viability was determined by the WST-1 assay. The slot blot and Western blot were used to detect intracellular Glycer-AGEs, and their localization was analyzed by confocal microscopy. Real-time reverse transcription-polymerase chain reaction was used to quantify the mRNA for the acute phase reactant C-reactive protein (CRP).
Results
Glyceraldehyde (GA), which is the precursor of Glycer-AGEs, induced a concentration- and time-dependent increase in cell death, which was associated with an increase in intracellular Glycer-AGEs formation. Aminoguanidine (AG), which prevents AGEs formation, inhibited the formation of intracellular Glycer-AGEs and prevented cell death. Among the intracellular Glycer-AGEs that were formed, heat shock cognate 70 (Hsc70) was identified as a GA-modified protein, and its modification reduced the activity of Hsc70. Furthermore, intracellular Glycer-AGEs increased the CRP mRNA concentration.
Conclusions
These results suggest that intracellular Glycer-AGEs play important roles in promoting inflammation and hepatocellular death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term nonalcoholic steatohepatitis (NASH) was initially coined by Ludwig et al. [1] to describe histopathological findings typical of alcoholic liver disease in patients without a history of significant alcohol abuse. NASH is one of a group of nonalcoholic fatty liver diseases (NAFLDs) that range from simple steatosis to steatohepatitis [2]. However, although simple steatosis appears to be a benign and nonprogressive condition, NASH is recognized as a potentially progressive disease that may cause cirrhosis, liver failure, and hepatocellular carcinoma [3, 4]. NASH is also recognized as a component of metabolic syndrome and is associated with insulin resistance and abnormalities in glucose and lipid metabolism [5–7]. The development of NASH appears to involve two pathophysiologic hits, with the first hit being steatosis, and the second hit being oxidative stress, decreased hepatic ATP production, and the induction of pro-inflammatory cytokines producing steatohepatitis [8]; however, the pathogenesis of NASH remains poorly understood.
Advanced glycation end-products (AGEs) are formed by the Maillard reaction, a nonenzymatic reaction between the ketones or aldehydes of sugars and the amino groups of proteins, which contributes to aging and to the pathological complications of diabetes [9, 10]. Recent studies have suggested that AGEs can arise not only from sugars but also from carbonyl compounds derived from the autoxidation of sugars and other metabolic pathways [11, 12]. Among different AGEs, there is evidence that glyceraldehyde-derived AGEs (Glycer-AGEs) are associated with such cytotoxicity [13–15].
We have demonstrated that Glycer-AGEs are elevated in the sera of patients with NASH. Furthermore, immunohistochemical staining of Glycer-AGEs showed intense staining in the livers of patients with NASH [16]. These observations led us to speculate that extracellular and intracellular Glycer-AGEs may play roles in the pathogenesis of NASH. In fact, the interaction of Glycer-AGEs with the receptor for AGEs (RAGE) was found to increase the expression of C-reactive protein (CRP) in hepatocellular carcinoma (Hep3B) cells [17] as well as the expression of transforming growth factor-β and monocyte chemoattractant protein-1 in a hepatic stellate cell line (LI90) [18]. In the present study, we examined the effects of intracellular Glycer-AGEs on cultured Hep3B cells.
Materials and methods
Chemicals
All chemicals were commercial samples of high purity and used as supplied. Glyceraldehyde (GA) was purchased from Nakalai Tesque (Kyoto, Japan) and aminoguanidine (AG) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of glyceraldehyde-derived AGEs and anti-Glycer-AGEs antibody
Glycer-AGEs-bovine serum albumin (BSA) was prepared as described previously [19]. Briefly, 25 mg/ml of BSA (A0281; Sigma-Aldrich) was incubated at 37°C for 7 days under sterile conditions with 0.1 M GA and 5 mM diethylenetriamine-pentaacetic acid (Dojindo Laboratories, Kumamoto, Japan) in 0.2 M phosphate buffer (pH 7.4). The modified albumin was purified by a PD-10 column (GE Healthcare UK, Buckinghamshire, England) chromatography and dialysis against phosphate-buffered saline (PBS). Protein concentrations were determined with the Dc protein assay reagent (Bio-Rad Laboratories, Richmond, CA, USA) using BSA as a standard.
Anti-Glycer-AGEs antibody was prepared and purified as described previously [19]. Briefly, 20 ml of stock rabbit antiserum was applied to a Glycer-AGEs-BSA-Sepharose 4B column. After extensive washing with PBS, the adsorbed fractions were eluted with 20 mM sodium phosphate buffer containing 2 M potassium thiocyanate (pH 7.4). The anti-Glycer-AGEs antibody fractions were pooled, concentrated using Centriprep-10 (Millipore, Billeria, MA, USA), and passed through a PD-10 column. The anti-Glycer-AGEs antibody thus obtained was loaded onto an N-(carboxymethyl)lysine-BSA-Sepharose 4B column, which was washed with PBS to obtain the unadsorbed fraction (anti-Glycer-AGEs antibody). The unadsorbed fractions were pooled, concentrated with Centriprep-10, and passed through a PD-10 column for use in this study.
Cell cultures
Hep3B cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX, USA) under standard cell culture conditions (humidified atmosphere, 5% CO2, 37°C). The cells (1.5 × 104 cells/cm2) were then seeded in various plates or culture dishes (Becton-Dickinson, Franklin Lakes, NJ, USA) and incubated for 24 h before the start of all experiments.
The GA and AG treatments were carried out in 2% FBS/DMEM. The cells were incubated with various concentrations of GA for 24 h or 4 mM GA for 3–24 h. To investigate the specific effects of AGEs, cells were preincubated with or without 16 mM AG for 2 h and then additionally incubated with GA.
Cell viability and apoptosis assay
Cell viability was determined by the WST-1 assay, which measures metabolic activity. After removing the medium from a 96-well microplate that had been used to culture Hep3B cells as stated above, 100 μl/well of 10% FBS/DMEM and 10 μl/well of WST-1 solution [5 mM WST-1, 0.2 mM 1-methoxy-5-methylphenazinium, and 20 mM hydroxyethylpiperazine ethanesulfonic acid (HEPES) (pH 7.4); Dojindo Laboratories] were added, and the cells were incubated for 2 h. Absorbance was then measured at 450 and 650 nm using a microplate reader (Labsystems multiskan ascent, Model No. 354; Thermo Fisher Scientific, Kanagawa, Japan). The net difference (A 450–A 650) was used as a measure of cell viability. Apoptosis was determined by a cell death detection enzyme-linked immunosorbent assay (ELISA; Roche, Mannheim, Germany), which measures DNA fragments. All procedures were performed according to the manufacturers’ instructions. The results were corrected for differences in cell number.
Preparation of cell lysate and slot blot analysis
The cells were washed with ice-cold Ca2+ and Mg2+ free PBS [PBS (−)] and subjected to lysis buffer [1% TritonX-100/Nonidet P-40, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 5 mM sodium pyrophosphate, 2 mM ethyleneglycoltetraaceticacid (EGTA), 5 mM ethylenediaminetetraacetate (EDTA), and 1× protease inhibitor cocktail (complete, Mini; Roche)]. Subsequently, the cell lysates were incubated on ice for 5 min and centrifuged at 10,000g for 10 min at 4°C. Protein concentrations were measured using the Bradford assay (Bio-Rad Laboratories).
The resultant cell lysates (30 μg of proteins/well) were then blotted under vacuum conditions on polyvinylidene difluoride (PVDF; ATTO, Tokyo, Japan) membranes using a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories). A biotinylated protein ladder marker (Cell Signaling, Beverly, MA, USA) was used as a positive control. Next, the membranes were blocked for 1 h at room temperature using 4% skimmed milk in PBS containing 0.05% polyoxyethylene sorbitan monolaurate (PBS-T). After being washed twice with PBS-T, the membranes were incubated with rabbit anti-Glycer-AGEs antibody at a dilution of 1:250 for 1.5 h. Subsequently, the membranes were washed twice with PBS-T and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Cell Signaling) at a dilution of 1:2,000 and HRP-conjugated anti-biotin antibody (Cell Signaling) against the biotinylated marker at a dilution of 1:1,000 for 1 h. After being washed a further two times with PBS-T, the immunoreactive proteins were detected with ECL Western Blotting Detection Reagents (GE Healthcare UK) using a luminescent image analyzer (LAS-1000UVmini; Fujifilm, Tokyo, Japan). The density of the bands was analyzed using a Multi Gauge version 3.0 (Fujifilm). The amount of Glycer-AGEs was calculated based on a standard curve for Glycer-AGEs-BSA, and the results were expressed as arbitrary units (U): 1 U corresponds to 1 μg of Glycer-AGEs-BSA. A positive control was used to correct for differences in density between membranes.
Neutralization of rabbit anti-Glycer-AGEs antibody
The amount of Glycer-AGEs-BSA required to neutralize rabbit anti-Glycer-AGEs antibody was calculated based on a standard curve of Glycer-AGEs-BSA in a competitive ELISA. The antibody was incubated with 100 μg of Glycer-AGEs-BSA for 1 h at room temperature and used for the subsequent experiments.
Immunofluorescence staining
Cells grown on glass coverslips in a 6-well plate were washed with ice-cold PBS (−) and fixed in methanol/acetone (1:1) for 5 min at room temperature. After being washed three times with PBS (−), the cells were blocked for 30 min using 4% skimmed milk in PBS-T, before being incubated with rabbit anti-Glycer-AGEs antibody at a dilution of 1:200 for 1 h. Subsequently, the cells were washed three times with PBS (−) and incubated with highly cross-adsorbed Hilyte Fluor 555-labeled goat anti-rabbit IgG antibody (AnaSpec, San Jose, CA, USA) at a dilution of 1:500 for 1 h. After being washed a further three times with PBS (−), the coverslips were mounted on glass microscope slides using VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA, USA) containing Hoechst 33258 solution (Dojindo Laboratories) to label the nucleus, and confocal microscopy was performed with a scanning confocal microscope system (LSM 510; Carl Zeiss, Tokyo, Japan).
Western blot analysis
Cell lysates (30 μg of proteins/lane) dissolved in sodium dodecyl sulfate (SDS) sample buffer [62.5 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, and 0.01% bromophenol blue] containing 5% 2-mercaptoethanol were boiled for 3 min at 95°C and separated by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto PVDF membranes. A biotinylated marker was used as a molecular weight marker. The detection of immunoreactive proteins was performed as described above. The primary antibodies used were: rabbit anti-Glycer-AGEs antibody at a dilution of 1:250, mouse anti-human heat shock cognate 70 (Hsc70) antibody (Santa Cruz, Santa Cruz, CA, USA) at a dilution of 1:200, and rabbit anti-human β-actin antibody (Cell Signaling) at a dilution of 1:500. The secondary antibodies used were: HRP-conjugated goat anti-rabbit IgG antibody at a dilution of 1:2,000, HRP-conjugated goat anti-mouse IgG antibody (Biosource, Camarillo, CA, USA) at a dilution of 1:5,000, and HRP-conjugated anti-biotin antibody at a dilution of 1:1,000.
In immunoprecipitation, the cell lysates (150 μg) were incubated for 1 h at 4°C with 2 μg of anti-human Hsc70 antibody and were captured by 40 μl of Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA) for 1 h at 4°C. The tubes were then placed on a magnet (Dynal MPC-S; Invitrogen) for 2 min to allow collection of the immunoprecipitates, which were subsequently washed three times with PBS (−), boiled with the SDS sample buffer containing 10% 2-mercaptoethanol for 3 min at 95°C, and subjected to Western blot analysis.
Real-time reverse transcription-polymerase chain reaction analysis
Total RNA was isolated from cells with ISOGEN (Nippon Gene, Tokyo, Japan), and 50 ng of RNA was reverse transcribed into cDNA with the PrimeScript RT reagent kit (Takara, Shiga, Japan) using a GeneAmp PCR System 9700 (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed with SYBR Premix Ex Taq (Takara) using a Smart Cycler II System (Takara). The reaction mixture (25 μl) contained 1× SYBR Premix Ex Taq, 0.2 μM PCR forward primers, 0.2 μM PCR reverse primers, and 10 ng of cDNA as template. Primer information is provided in Table 1. All processes were performed according to the manufacturers’ instructions. The expression levels of target genes were calculated using a relative quantification method. Briefly, standard curves were prepared for the target and control genes using serial dilutions of a cDNA of known concentration as a template. β-Actin was used as an endogenous control gene in order to normalize the target gene expression values. Product specificity was determined by melting curve analysis. Calculation of the correlation coefficient (r 2) of the linear regression line related to the standard curve was performed for each run, and the minimum value was 0.988.
Preparation of GA-modified Hsc70 and the chaperone assay
GA-modified Hsc70 was generated by the incubation of 3 μM recombinant human Hsc70 (StressMarq, Victoria, BC, Canada) with 2.1 mM GA in 20 mM phosphate buffer (pH 7.4) for 6 h at 37°C. Control Hsc70 was incubated in a similar manner in the absence of GA. Immediately post-incubation, the samples were subjected to an ultrafiltration spin column (Vivaspin, 10 k; Vivascience, Hannover, Germany) to remove any unreacted GA, and the protein fractions were collected.
QuantiLum Recombinant Luciferase (50 nM; Promega, Madison, WI, USA) was denatured for 15 min at 42°C in buffer A [25 mM HEPES (pH 7.4), 5 mM MgCl2, 150 mM KCl, 2 mM dithiothreitol (DTT), and 3 mM ATP] containing either 1.7 μM BSA or the chaperone proteins [1.7 μM Hsc70 or GA-modified Hsc70 and 2 μM Hsp40 (Stressgen, Victoria, BC, Canada)]. For renaturation, the denatured luciferase was diluted fivefold in buffer B [25 mM HEPES (pH 7.4), 5 mM MgCl2, 50 mM KCl, 2 mM DTT, 3 mM ATP, and 50% rabbit reticulocyte lysate (Promega)], and the reaction mixtures were incubated for 1 h at 30°C. Luciferase activity was measured using the luciferase assay system (Promega) and a luminometer (AB-2200; ATTO). An equal amount of native luciferase was measured and defined as 100%.
Statistical analysis
All experiments were performed in duplicate and repeated at least two to three times, with each experiment yielding essentially identical results. Data were expressed as mean ± SD. The significance of difference between group means was determined by one-way analysis of variance. P values <0.05 were considered to be statistically significant.
Results
Effect of glyceraldehyde on cell viability in Hep3B cells
We first examined the effect of GA on cell viability in Hep3B cells. Cell viability was determined by a WST-1 assay after 24 h incubation with GA. 1, 2, and 4 mM of GA significantly decreased cell viability to 81.1, 46.1, and 8.4%, respectively, of the control (Fig. 1a). Furthermore, apoptosis was determined by a cell death detection ELISA. Two and 4 mM of GA significantly increased apoptosis to 4.8 and 6.0 times, respectively, of the control (Fig. 1b).
Effects of glyceraldehyde (GA) in Hep3B cells. a Cell viability was determined by the WST-1 assay. Cells were preincubated with or without 16 mM of aminoguanidine (AG) for 2 h and additionally incubated with 0–4 mM of GA for 24 h. b Apoptosis was determined by cell death detection enzyme-linked immunosorbent assay (ELISA). Cells were incubated with 0–4 mM of GA for 24 h. Data are shown as the mean ± SD (a n = 6, b n = 3) **P < 0.01 vs 0 mM GA
We next investigated whether Glycer-AGEs were formed during GA-induced cell death. AG effectively inhibits the formation of AGEs in vitro and in vivo [20, 21], and it is thought to act via its chelating, antioxidant, and carbonyl trapping activities [22, 23]. The GA-induced decrease in cell viability was almost completely restored by pretreatment with 16 mM of AG (Fig. 1a).
GA enhanced the formation of intracellular Glycer-AGEs in Hep3B cells
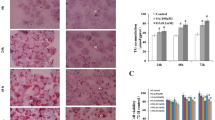
We next examined whether intracellular Glycer-AGEs were detected by slot blot analysis, and such products were evident at 24 h (Fig. 2a). In cells treated with 4 mM GA, Glycer-AGEs were strongly detected, whereas few immunoreactive Glycer-AGEs were detected after AG pretreatment. After incubation in 4 mM GA for 6 and 12 h, cell viability was significantly decreased, to 64.6 and 11.1%, respectively, of the control (data not shown). Intracellular Glycer-AGEs formation also increased during this period: treatment with 4 mM GA for 6, 12, and 24 h increased the amount of Glycer-AGEs to 6.9, 13.1, and 28.0 mU, respectively (Fig. 2b). Intracellular Glycer-AGEs formation was also prevented by AG pretreatment.
Slot blot analysis of intracellular glyceraldehyde-derived advanced glycation end-products (Glycer-AGEs). The cells were preincubated with or without 16 mM AG for 2 h and additionally incubated with 0–4 mM GA for 24 h (a) or 4 mM GA for 6–24 h (b). Cell lysates (30 μg of protein/well) were blotted under vacuum conditions onto polyvinylidene difluoride (PVDF) membranes, and the proteins were probed with anti-Glycer-AGEs antibody or neutralized anti-Glycer-AGEs antibody by an excess of Glycer-AGEs-bovine serum albumin (BSA). The amount of Glycer-AGEs was calculated based on a standard curve for Glycer-AGEs-BSA. One mU corresponds to 1 ng of Glycer-AGEs-BSA standard. c The cells were incubated in various concentrations of GA for 24 h. Immunofluorescence staining of the nucleus (Hoechst 33258, blue), Glycer-AGEs (HiLyte Fluor555, red), and merged images (pink) are shown. ×800. a 0 mM GA, b 1 mM GA, c 2 mM GA, d 4 mM GA
In immunofluorescence staining, intracellular Glycer-AGEs were evident after treatment with 2 or 4 mM GA and were localized in the cytosol and nucleus (Fig. 2c). In particular, after 4 mM GA treatment, these AGEs were stained strongly, and morphologic changes were also evident in the nucleus.
To investigate the composition of GA-modified proteins, intracellular Glycer-AGEs were analyzed by Western blot analysis. No intracellular Glycer-AGEs were detected after incubation with 1 mM GA for 24 h, but some were weakly detected at 73 kDa after treatment with 2 mM GA. After incubation with 4 mM GA, Glycer-AGEs with three different molecular weights were detected (84, 73, and 45 kDa). In addition, cross-linking and aggregation, which are characteristics of AGEs, were observed at high molecular weights, and no immunoreactivity was evident in the presence of the AGEs inhibitor AG (Fig. 3).
Western blot analysis of intracellular Glycer-AGEs. Cells were preincubated with or without 16 mM AG for 2 h and additionally incubated with 0–4 mM GA for 24 h. Cell lysates (30 μg of proteins/lane) were loaded onto a 10% polyacrylamide gel. Glycer-AGEs in the protein were determined by Western blot analysis using anti-Glycer-AGEs antibody. Size markers (kDa) are shown on the left. Equal protein loading was estimated using anti-β-actin antibody
Identification of the GA-modified proteins
With respect to the nature of the major 73 kDa species of GA-modified protein, we hypothesized that it was heat shock protein 70 (Hsp70), based on its molecular weight and past reports [24, 25]. The Hsp70 family consists of four proteins. The localization of glucose-regulated proteins 78 and 75 is confined to the endoplasmic reticulum and the mitochondria, respectively, whereas the constitutively expressed heat shock cognate (Hsc70) and the stress-inducible Hsp70 protein reside mainly in the cytosol and nucleus, respectively [26]. The appearance of Hsc70 was increased in a time-dependent fashion by 4 mM GA (Fig. 4a, right panel), whereas the level of Hsc70 mRNA was not changed by GA (Fig. 4b). These results provide evidence for the reduced degradation and the acquisition of protease tolerance by glycation. High-molecular-weight, Hsc70-containing complexes were also evident and their distribution closely resembled that of immunoreactive Glycer-AGEs (Fig. 4a, left panel).
Identification of GA-modified proteins. a Cells were incubated with 4 mM GA for 6–24 h. Cell lysates (30 μg of proteins/lane) were loaded onto a 10% polyacrylamide gel, and the Glycer-AGEs in the protein were determined by Western blot analysis using anti-Glycer-AGEs antibody (left panel) or anti-heat shock cognate (Hsc70) antibody (right panel). Equal protein loading was determined using anti-β-actin antibody. b Cells were incubated with 4 mM GA for 3 or 6 h. The levels of mRNA expression were analyzed by a real-time reverse transcription-polymerase chain reaction (RT-PCR) method, and the result was normalized to the β-actin mRNA level. Data are shown as mean ± SD (n = 3). c Cells were incubated with 4 mM GA for 24 h. Western blot analysis was performed on the immunoprecipitates after Hsc70 immunoprecipitation. The proteins were probed with anti-Glycer-AGEs antibody or neutralized anti-Glycer-AGEs antibody (upper panel) and anti-Hsc70 antibody (lower panel). Left panel cell lysate. Middle and right panels immunoprecipitate
To identify GA-modified proteins, Hsc70 was immunoprecipitated with anti-Hsc70 antibody and probed with an anti-Glycer-AGEs antibody. Glycer-AGEs were detected at 73 kDa as well as in the high-molecular-weight complexes (Fig. 4c).
Effects of GA modification on the chaperone activity of Hsc70
We generated GA-modified Hsc70 by incubating Hsc70 with GA and confirmed AGEs modification with an anti-Glycer-AGEs antibody. Unmodified Hsc70 was not detected under these conditions. Furthermore, GA-modified Hsc70 was present in high-molecular-weight complexes (Fig. 5a), which was similar to the pattern observed in the cell lysates (Fig. 4a, right panel).
Effects of glyceraldehyde modification on the chaperone activity of Hsc70. a Three μM Hsc70 was incubated with or without 2.1 mM GA for 6 h at 37°C. The samples (0.75 μg of proteins/lane) were loaded onto a 10% polyacrylamide gel and probed with anti-Glycer-AGEs antibody (left panel) and anti-Hsc70 antibody (right panel). b Luciferase was denatured for 15 min at 42°C in the presence of 1.7 μM BSA, Hsc70, or GA-modified Hsc70. The luciferase activity was measured after 1 h incubation at 30°C with 50% reticulocyte lysate. The mean of three independent measurements is given. Data are shown as mean ± SD **P < 0.01 vs B, # P < 0.05 vs H. H Hsc70, G GA-modified Hsc70, B BSA
The functional effect of the modification of Hsc70 by GA was assessed by measuring the extent of luciferase refolding. Luciferase was denatured in the presence of BSA, Hsc70, or GA-modified Hsc70. In the presence of BSA, the addition of reticulocyte lysate led to a 19% recovery in luciferase activity after 1 h. The presence of Hsc70 led to a significant restoration of luciferase activity to 39% of the control, whereas GA-modified Hsc70 only restored the activity to 26% of the control (Fig. 5b). Thus, GA modification reduced the functional activity of Hsc70 with respect to protein refolding.
Effect of intracellular Glycer-AGEs on the mRNA expression of CRP
Finally, to investigate whether GA modification influenced the inflammatory function of hepatocytes, which is a prominent feature of NASH, we examined the expression of CRP mRNA. The expression of CRP mRNA was significantly increased by 4 mM GA at 6 h (Fig. 6a), whereas it was significantly suppressed by pretreatment with the AGEs inhibitor AG (Fig. 6b).
Effects of intracellular Glycer-AGEs on the mRNA expression of C-reactive protein (CRP). a Cells were incubated with 4 mM GA for 3 or 6 h. b Cells were preincubated with or without 16 mM AG for 2 h and additionally incubated with 4 mM GA for 6 h. The levels of mRNA expression were analyzed by a real-time RT-PCR method, and the result was normalized to the β-actin mRNA level. Data are shown as mean ± SD (n = 3) *P < 0.05, **P < 0.01 vs 0 mM GA, ## P < 0.01 vs 4 mM GA
Discussion
Insulin resistance and diabetes mellitus (DM) are common features of the metabolic syndrome associated with obesity and probably contribute to comorbid liver disease. Several recent studies have indicated an enhanced prevalence and severity of liver disease in diabetic patients [27, 28]. However, the susceptibility to NASH in diabetic patients and its precise mechanisms have not been fully addressed.
One potential mechanistic link between DM and NASH involves AGEs. The formation of AGEs is enhanced during DM, and compared to healthy controls or patients with simple steatosis, serum and hepatic Glycer-AGE levels are significantly elevated in patients with DM [16]. The interaction of Glycer-AGEs with the AGEs receptor, RAGE, has been linked to the induction of oxidative stress, as well as the enhanced fibrogenic potential of cultured human stellate cells [18]. Thus, Glycer-AGEs signaling via RAGE and the resulting generation of reactive oxygen intermediates may contribute to the increase in hepatic pathology found in diabetic patients.
The present studies were designed to examine the hypothesis that the formation of intracellular Glycer-AGEs, induced by an excess intracellular concentration of GA due to abnormalities in glucose and fructose metabolism, might contribute to the development of NASH. We observed that GA induced concentration- and time-dependent cell death and increased the intracellular concentration of Glycer-AGEs in Hep3B cells. AG, which is an inhibitor of AGEs formation, reduced the formation of intracellular Glycer-AGEs and prevented GA-induced cell death (Figs. 1a, 2a, b). Furthermore, when cells were cultured in 2% FBS/DMEM, GA did not increase the extracellular Glycer-AGEs concentration (data not shown). Consequently, the formation of intracellular Glycer-AGEs is associated with cell death. In addition, this cell death occurred via apoptosis (Fig. 1b). Of note, some authors have reported that hepatocyte apoptosis is a prominent feature of NASH [29, 30].
In the liver, GA, which is a precursor of Glycer-AGEs, is considered to be formed by two pathways: (1) the glycolytic pathway and (2) the fructose metabolic pathway [31]. (1) In glycolysis, the intermediate glyceraldehyde-3-phosphate (G-3-P) is metabolized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH), eventually forming pyruvate. With a decline in GAPDH activity, G-3-P accumulates intracellularly. G-3-P metabolism then shifts to another route, and the amount of GA is increased. (2) Fructose metabolism involves fructokinase and is especially important in the liver after a meal. Fructose is phosphorylated to fructose-1-phosphate (F-1-P) by a specific kinase, and the liver aldolase aldolase B can cleave F-1-P. In this case, the products are dihydroxyacetone phosphate and GA. Therefore, the liver more readily accumulates GA than other organs.
Whether the in vitro addition of GA to hepatocytes is physiologically relevant is an important issue for further study. Taniguchi et al. [32] demonstrated that islets exposed to 20 mM glucose accumulated 0.025 pmol/islet GA, whereas exposure to 10 mM GA caused an accumulation of 0.12 pmol/islet GA. Based on this report, Takahashi et al. [33] used 2 mM GA in their experiments, which is a concentration similar to that observed using 20 mM glucose. Indeed, we observed that 2 mM GA markedly increased the intracellular concentration of Glycer-AGEs. In addition, it also has been reported that long-term exposure to high glucose concentrations decreases GAPDH activity in islets, which could lead to excess GA accumulation [34].
We showed that a Glycer-AGEs modified protein of 73 kDa, which we identified to be Hsc70, was detected earliest and in greatest abundance in GA-treated hepatocytes (Figs. 3, 4, 5). This Glycer-AGEs may play a critical role in GA-induced cytotoxicity, and it is well known that AGEs modification adversely alters protein function [35, 36].
It has been previously reported that the Hsp family of proteins are readily modified by reducing sugars [37, 38], and Hsp70 has been identified as a methylglyoxal-modified protein in yeast [38]. Furthermore, the post-translational modification (tyrosine nitration) of Hsc70 and Hsp70 by d-galactosamine has been shown to participate in the induction of apoptosis and necrosis in cultured human hepatocytes [25]. Hsc70 and Hsp70 act as molecular chaperones that assist in the proper folding, assembly, and intracellular trafficking of newly synthesized proteins, as well as in the regulation of cellular homeostasis and the promotion of cell survival [26]. We also demonstrated that GA modification reduced the ability of Hsc70 to refold denatured luciferase. These data, taken together, suggest that Hsc70 loss of function by glycation may induce cell death.
Finally, we showed that intracellular Glycer-AGEs increased the expression of the mRNA of the acute phase reactant CRP (Fig. 6). Recent studies have shown that the concentration of high-sensitivity CRP is elevated in the plasma of patients with NASH compared to that in patients with simple steatosis and that in healthy subjects [39, 40]. The severity of liver histology in NASH patients was strongly associated with increasing plasma hs-CRP concentration [40], and it was suggested that intracellular Glycer-AGEs may trigger an inflammatory response, which is one of the prominent features of NASH.
In summary, we demonstrated that the formation of intracellular Glycer-AGEs induced hepatocyte cell death and triggered an acute phase inflammatory response. These results suggest that intracellular Glycer-AGEs may play a critical role in the pathogenesis of NASH and may serve as potential targets for therapeutic intervention.
References
Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31.
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–5.
Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50.
Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–9.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5.
Al-Abed Y, Kapurniotu A, Bucala R. Advanced glycation end products: detection and reversal. Methods Enzymol. 1999;309:152–72.
Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101.
Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–26.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16.
Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, et al. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000;59:1094–105.
Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, et al. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290:973–8.
Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, et al. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem. 2002;277:20309–15.
Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112–9.
Yoshida T, Yamagishi S, Nakamura K, Matsui T, Imaizumi T, Takeuchi M, et al. Pigment epithelium-derived factor (PEDF) inhibits advanced glycation end product (AGE)-induced C-reactive protein expression in hepatoma cells by suppressing Rac-1 activation. FEBS Lett. 2006;580:2788–96.
Iwamoto K, Kanno K, Hyogo H, Yamagishi S, Takeuchi M, Tazuma S, et al. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol. 2008;43:298–304.
Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med. 2000;6:114–25.
Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–32.
Nicholls K, Mandel TE. Advanced glycosylation end-products in experimental murine diabetic nephropathy: effect of islet isografting and of aminoguanidine. Lab Invest. 1989;60:486–91.
Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999;257:251–8.
Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–72.
Carbone DL, Doorn JA, Kiebler Z, Sampey BP, Petersen DR. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem Res Toxicol. 2004;17:1459–67.
Rodríguez-Ariza A, López-Sánchez LM, González R, Corrales FJ, López P, Bernardos A, et al. Altered protein expression and protein nitration pattern during d-galactosamine-induced cell death in human hepatocytes: a proteomic analysis. Liver Int. 2005;25:1259–69.
Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10.
Canbakan B, Senturk H, Tahan V, Hatemi H, Balci H, Toptas T, et al. Clinical, biochemical and histological correlations in a group of non-drinker subjects with non-alcoholic fatty liver disease. Acta Gastroenterol Belg. 2007;70:277–84.
Bell DS, Allbright E. The multifaceted associations of hepatobiliary disease and diabetes. Endocr Pract. 2007;13:300–12.
Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and FAS expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43.
Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–17.
Takeuchi M, Yamagishi S. Alternative routes for the formation of glyceraldehyde-derived AGEs (TAGE) in vivo. Med Hypotheses. 2004;63:453–5.
Taniguchi S, Okinaka M, Tanigawa K, Miwa I. Difference in mechanism between glyceraldehyde- and glucose-induced insulin secretion from isolated rat pancreatic islets. J Biochem. 2000;127:289–95.
Takahashi H, Tran PO, LeRoy E, Harmon JS, Tanaka Y, Robertson RP. d-Glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective beta cell function via non-mitochondrial pathways. J Biol Chem. 2004;279:37316–23.
Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300:216–22.
Hamelin M, Mary J, Vostry M, Friguet B, Bakala H. Glycation damage targets glutamate dehydrogenase in the rat liver mitochondrial matrix during aging. FEBS J. 2007;274:5949–61.
Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–8.
Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006;580:1565–70.
Gomes RA, Miranda HV, Silva MS, Graça G, Coelho AV, Ferreira AE, et al. Yeast protein glycation in vivo by methylglyoxal. Molecular modification of glycolytic enzymes and heat shock proteins. FEBS J. 2006;273:5273–87.
Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–82.
Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16:1394–9.
Acknowledgments
This work was supported in part by grants from the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research (B), #19300254).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takino, Ji., Kobayashi, Y. & Takeuchi, M. The formation of intracellular glyceraldehyde-derived advanced glycation end-products and cytotoxicity. J Gastroenterol 45, 646–655 (2010). https://doi.org/10.1007/s00535-009-0193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0193-9