Abstract
Introduction
Pancreatic carcinoma has one of the poorest prognoses among malignant tumors. Many pancreatic carcinoma patients who undergo common treatments, such as surgery, radio-chemotherapy and chemotherapy, gained little benefit because of the histological characteristics.
Materials and methods
HIFU is a new technique of noninvasive treatment for unresectable pancreatic carcinoma. HIFU has the ability to ablate the deep tissues inside body from an external source using high-intensity focused ultrasound. The effects of HIFU can result in cell destruction and tissue necrosis.
Results
Results from study in China in 251 patients with advanced pancreatic carcinoma suggested that HIFU treatment could reduce the size of tumors without causing complications and prolong survival. Moreover, according to some reports from China, HIFU treatment is suggested to be useful as the one of palliative treatments for unresectable pancreatic carcinoma. Our case of HIFU therapy for pancreatic carcinoma is presented including pathological findings in this paper. The results suggested that HIFU treatment might be effective in controlling local tumor.
Conclusion
HIFU therapy may have the possibility of becoming one of the combination therapies for treating pancreatic carcinoma in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The yearly mortality due to pancreatic carcinoma exceeds 200,000 [1] worldwide and is increasing. Even with recent advances in diagnostic imaging technology, most cases of pancreatic carcinoma are only discovered at an unresectable stage, when the prognosis is poor and the 5-year survival rate is <1% [1]. To date, multimodality therapy, consisting of chemotherapy with 5-fluorouracil (5-FU) [2] and radiotherapy [3], has been the standard treatment for unresectable pancreatic carcinoma. Since the results of these treatments were unsatisfactory, gemcitabine (GEM) (Eli Lilly Japan, Kobe, Japan) has recently become the standard chemotherapy medication for unresectable pancreatic carcinoma, and GEM is expected to both increase antitumor efficacy [4] and improve clinical benefit response (CBR) [4]. However, it cannot be said that the results of chemotherapy are sufficient for unresectable pancreatic carcinoma. Therefore, new advances in therapy are required for these patients.
Recently, the technique of ultrasound has been developed. Its use has spread not only to diagnostic imaging and needle guidance or biopsy for percutaneous procedures but also to directed therapy for tumors. Ultrasound technology has been developed to use focused ultrasound energy for therapeutic purposes, such as tissue ablation. High-intensity focused ultrasound (HIFU) is being promoted as a method to ablate the tumor and achieve pain relief in unresectable pancreatic carcinoma. HIFU therapy is regarded as minimally invasive or noninvasive therapy and has few side effects, unlike radiofrequency therapy. Therefore, it may be possible to combine HIFU with chemotherapy as a minimally invasive combination therapy.
This article provides a summary of HIFU, that is, the principles, method, effects, cases of HIFU therapy, and current clinical application for pancreatic carcinoma.
The principles of HIFU
HIFU has the ability to deliver high-intensity focused ultrasound from an external source into deep tissues, with a large convergence angle. The combination of thermal, mechanical (cavitation), and biological effects can result in cell death and tissue necrosis [5]. The device is used clinically to treat both benign and malignant solid tumors [5]. HIFU differs from the traditional hyperthermal therapy. The results of previous animal experiments have shown that transient temperatures were 70–100°C at the focal point inside the body [6]. Results presented in this paper showed that HIFU can destroy tumors or restrain the growth of tumor tissue. The hyperthermia due to HIFU (which can reach temperatures higher than 70°C in the targeted area) causes necrosis, liquefaction, absorption, and fibrosis of tissues [5], which could bring about the effects of HIFU thermal therapy.
Clinical history of HIFU
Lindstrom [7] and Fry et al. [8] investigated the possibility of using high-intensity ultrasound for therapy of neurological disorders in the 1950s. Subsequently, ultrasonic therapy for tumors was investigated in the 1970s, although using only low-intensity ultrasound [9]. The concept was to produce hyperthermia within the tumor, maintaining the elevated temperature for a certain period of time. However, this method lacked sufficient control to reach and maintain a consistent temperature within the tumor. The first clinical application which used high-intensity ultrasound was in the treatment of kidney stones using extracorporeal shockwave lithotripsy (ESWL) in the 1980s [10]. The development of technology such as magnetic resonance thermometry brought about the innovation of HIFU for the treatment of tumors in the 1990s. Moreover, it has been recognized that HIFU can give rise to almost immediate cell death by coagulation necrosis in selected regions of tissue, raising the possibility of a new treatment for tumors [11–13].
New kinds of HIFU systems have been developed by several companies recently. HIFU systems fall into two types of method: bombardment from outside the body and transendothelial rectum bombardment.
Those systems using bombardment from outside the body include Haifa (Insightec, Ltd, Israel), CZ901 (Mianyang Sonic Electronic, China), Chongqing HIFU (Chongqing HIFU, China), Sonablate 500 (Focus Surgery, Inc., USA), FEB-BY Series HIFU System (China Medical Technologies, Inc., China). Transendothelial rectum bombardment is used by EDAP (Edap Technomed Inc., France). Of these, it is expected that the FEB-BY Serial HIFU System (China Medical Technologies, Inc., China) is the HIFU system which will receive the most attention.
Characteristics of the FEB-BY Series HIFU therapy system
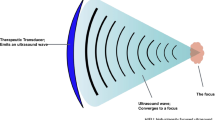
The CMT FEP-BY Series HIFU therapy system (Fig. 1) has the capacity to deliver high-intensity focused ultrasound from an external source deep into tissues, with a large convergence angle. The focus is oval in shape and is approximately 3 mm in diameter and 10 mm in length. The combination of thermal and mechanical (cavitation) biological effects can result in cell death and tissue necrosis. The device is clinically used to treat both benign and malignant solid tumors.
The main characteristics of this system are as follows: (1) Noninvasive procedure: treatment performed with this HIFU therapy system does not require incisions or transfusions, thus minimizing the risks and complications associated with invasive procedures. (2) Minimal side-effects: the large aperture of the transducer produces less intense ultrasound waves at the point of skin penetration, significantly reducing the likelihood of skin burns and collateral damage to healthy body tissue. (3) High degree of safety: treatment can be performed without anesthesia and does not cause significant discomfort, skin-burn, or hemorrhage. (4) Treatment for a broad range of tumors: the two-transducer system enables the HIFU therapy system to target a wide range of tumors including those of the liver, breast, kidney, pancreas and so on.
This instrument also has some advantages in comparison with those of other companies. These include ultrasound imaging guidance, no anesthesia needed, two transducers, spherical direct focus, and temperature monitoring. Furthermore, this device has been recently updated to add some new functions, such as a temperature monitoring system, a respiratory monitoring system, three-dimensional treatment planning, and real-time monitoring. Monitoring the focal temperature produces more effective therapy and is achieved by analyzing the echo signals acquired by the system before and after HIFU exposure. It reduces the targeting of unintended tissue caused by movement. The device automatically analyzes the respiratory status of the patient using real-time B-mode ultrasound images; allows consistent delivery of HIFU energy during either end expiration or inspiration; allows for accurate targeting of the tumor regardless of the geometry; and provides B-mode images of the treatment plane which are captured between pulse emissions.
The characteristics of the HIFU transducer are as follows (Fig. 2): frequency 1.1 MHz; aperture diameter 37 cm; radius of curvature 25.5 cm; and 251-element array. Each ceramic piezoelectric unit is selected from tens of thousands of units. In order to achieve high focusing accuracy and stability, each transducer is made of hundreds of elements with the same frequency. The wide angle of convergence (80°) of the high-intensity ultrasound beam keeps the acoustic intensity at the skin surface below the threshold for pain and skin burns. A large-aperture transducer creates a wide convergent angle to decrease the acoustic intensity at skin level, avoiding skin burn.
The HIFU therapeutic unit has an output power of 1–2 kW, an effective treatment depth of 3.5–14.0 cm, a therapy medium of degassed water, a practical focused volume of 0.3 cm × 0.3 cm × 0.8 cm (Fig. 3), and an effective focused volume of 0.6 cm × 0.6 cm × 1.0 cm. According to one report [14], the point accumulation method was applied to form the treatment matrix. Larger tumors can be fully treated by dividing them into several treatment segments. Energy is delivered in a pulsed mode. Treatment parameters are: transmit time (t1), interval time (t2), input power, and number of pulses. Primary parameters are: output power of 1–2 kW; transmit time (t1) of 0.1–0.28 s; an interval between shots of t2, t1:t2 = 2:1; and number of treatment shots at each spot (T) of 30–80 times. All of the parameters can be properly adjusted according to the location and depth of the tumor, the density of the tumor tissue, and the attenuation of ultrasound.
Principles of the HIFU therapy system. The CMT FEP-BY Series system has the ability to deliver high-intensity focused ultrasound from an external source into deep tissues, with a large convergence angle. The focus is oval in shape and is approximately 3 mm in diameter and 10 mm in length. Transducers have multi-element array and concave focusing. The patented multi-element array technology ensures an even acoustic field. The angle of convergence is 80° and the size of the focal point is smaller than 3 mm × 3 mm × 10 mm
Case presentation
The treatment of our first case of unresectable pancreatic carcinoma at our institution is presented below, including the practical methods.
The patient had multiple episodes of acute pancreatitis since August 2006. He also had a lower bile duct stricture, and was admitted to our institution for diagnosis. His diagnosis was autoimmune pancreatitis with related sclerosing cholangitis, and he was checked regularly. A mass of 30 mm was seen in the pancreas body on CT imaging in September 2007; no mass had been seen on CT in July. The mass was diagnosed as pancreatic carcinoma by percutaneous biopsy. The tumor was inoperable because of invasion of the superior mesenteric artery (SMA). After adequate intensive care, chemotherapy in combination with HIFU was performed.
Chemotherapy began on November 7, 2008. HIFU therapy was performed on December 1, 2008.
None of the patient’s laboratory data were abnormal apart from minimal tumor marker elevation. Physical findings were normal. The ultrasound (US) images (B-mode and contrast-enhanced ultrasonography; CE-US) and CT images are shown in Figs. 4 and 5. The tumor was visualized as a hypo-echoic mass near the superior mesenteric vein (SMV) in B-mode US. After chemotherapy but before HIFU therapy, CE-US showed an increase in blood flow inside the tumor though CE-US imaging had shown only hypovascularity before chemotherapy [15]. This changed image was used for comparison with the images after HIFU therapy. CT imaging demonstrated that the tumor had low density (Fig. 5).
The position, size, and morphological aspects of the tumor, and the tumor’s relationship to the adjacent organ were determined from imaging equipment before treatment. The intestinal cavity was cleaned by the patient fasting, taking oral laxative medication, and drinking degassed water. During treatment the patient lay on a treatment table facing down. Echo jelly was painted thickly over the bombardment area. B-mode ultrasound scanner monitoring system was used to define the treatment range, treatment layers, and power, and then the treatment probe was moved according to the planned procedure (Fig. 6).
HIFU therapy was performed four times over the course of 10 days, each treatment lasting about an hour and a half (Fig. 7). Treatment parameters were as follows: transmit power 500–1,000 W; 200 × 3–4 pulses/spot; transmit time (t1) 150–1,000 ms; interval time (t2) 150–3,000 ms; 47 treatment points in total. Anesthesia and analgesic drugs were not required during treatment. No accidents or complications (including skin-burn) occurred.
After HIFU therapy, no apparent change was seen on CT imaging (Fig. 8). However, B-mode US revealed hyper-echoic changes in the area of HIFU therapy (Fig. 9). CE-US imaging also revealed some more interesting changes. Isovascularity of the tumor before HIFU therapy had changed to hypovascularity in the area of HIFU therapy, that is, the blood flow of tumor inside increasing before HIFU therapy was decreased after HIFU therapy (Fig. 10).
Percutaneous biopsy was performed after treatment of HIFU about 1 month later. The results of histopathology, using HE and immunostaining imaging, revealed a few viable carcinoma cells inside the tumor . However, there was no finding of increasing in tumor and liver metastases in temporal images. Unfortunately, this patient died from carcinomatous peritonitis in our affiliated hospital 10 months after HIFU treatment. At autopsy, the tumor which had been treated by HIFU was replaced by a scar, and there was no apparent mass lesion in the area. The branch of large vessel through the tumor had not been destroyed (Fig. 11a–c). In addition, HE and immunostaining images only showed a very few viable tumor cells which were a little scattered (Fig. 11d, e). Furthermore, the vasculature of the branch of large vessel through the tumor had remained after HIFU treatment without being destroyed (Fig. 11e).
Autopsy images. a–c Macro-imaging: the area of tumor which had been treated by HIFU was replaced by a scar, and no apparent mass lesion was found in the area. The branch of large vessel through the tumor had not been destroyed. d, e Micro-imaging; HE images showed a very few viable tumor cells, which were a little scattered. Furthermore, the vasculature of the branch of large vessel running through the tumor was not destroyed
Discussion
HIFU is a new technique of noninvasive treatment for unresectable pancreatic carcinoma. HIFU has the ability to ablate deep tissues inside the body using high-intensity focused ultrasound from an external source. HIFU can cause cell destruction and tissue necrosis and differs from traditional hyperthermal therapy. Previous animal experiments have shown transient temperatures of between 70 and 100°C at the focal area inside the body [6]. According to this paper, HIFU can destroy tumors or restrain the growth of tumor tissue. Hyperthermia due to HIFU can reach temperatures higher than 70°C in the targeted area, which then causes necrosis within a few seconds, liquefaction, absorption, and fibrosis of tissues. The effects of HIFU [5] are thought to be thermal effects, mechanical effects, biological effects, coagulation necrosis, apoptosis, and nonlinear effects. Mechanical effects, such as cavitation, microstreaming, and radiation forces, are associated with high intensities. At the level of biological effects, high-intensity ultrasound can result in tissue heating and necrosis, cell apoptosis, and cell lysis. Coagulation necrosis occurs in tissue exposed to high-intensity ultrasound when the temperature of the tissue is elevated to a certain level for a certain time. Moreover, although most initial cell death in tissues exposed to HIFU is caused by cell necrosis from thermal injury, HIFU can also induce apoptosis. Nonlinear effects are observed at high acoustic intensities, resulting in faster attenuation of the ultrasound energy and faster tissue heating. The pathological findings of our case showed the fibrosis change of ablated tumor. This means that HIFU therapy can bring cell necrosis and apoptosis, and progress cell destruction and fibrosis. This suggested that HIFU treatment could be useful for local tumor control.
Pancreatic carcinoma is one of the most common malignant tumors. Treatment is difficult due to its fast progression. The median survival with pancreatic carcinoma is about 6 months. In addition, many pancreatic carcinoma patients who undergo common treatments, such as surgery, radio-chemotherapy, and chemotherapy, receive little benefit because of the histological characteristics. It seems that HIFU therapy may benefit local tumor control, as in our case. Treatment with HIFU will be useful for those patients who develop symptoms, and may become a palliative treatment for pancreatic carcinoma. Results of a study in China of 251 patients with advanced pancreatic carcinoma have suggested that HIFU treatment could reduce tumor size without causing complications, and prolong survival [16]. An interesting finding was that 84% of patients with pain due to pancreatic carcinoma obtained significant relief of their pain after treatment with HIFU. According to some reports from China [14, 17–23], HIFU treatment may be useful as a palliative treatment for unresectable pancreatic carcinoma. HIFU may be noninvasive and effective, but is a new technique and many aspects require inspection. Therefore, HIFU treatment cases should be gathered, and randomized controlled trials should be conducted to determine the results of HIFU treatment for pancreatic carcinoma in terms of local tumor response and clinically beneficial outcomes, e.g. by improving pain, functional status, quality of life, or survival.
In conclusion, the clinical applications of HIFU therapy have begun a new era of noninvasive local treatment. HIFU therapy may become one of the combination therapies for pancreatic carcinoma in the future.
References
World Health Organization. The world health report. Geneva: WHO; 2000.
Rothman H, Cantrell JE, Lokich J, et al. Continuous infusion of 5-fluorouracil plus weekly cisplatin for pancreatic carcinoma. Cancer. 1991;68:264–8.
The Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–5.
Burris HA, Moore Mj, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Dubinsky TJ, Cuevas C, Dighe MK, et al. High-intensity focused ultrasound: current potential and oncologic applications. AJR. 2008;190:191–9.
He S-X, Xiong L-L, Yao S-S, et al. The preclinical research of high intensity focused ultrasound. J Beijing Med Univ. 1999; 31:573–76.
Lindstrom PA. Prefrontal ultrasonic irradiation: a substitute for lobotomy. Arch Neurol Psych. 1954;72:399–425.
Fry W, Barnard J, Fry F, Krumins R, Brennan J. Ultrasonic lesions in the mammalian central nervous system with ultrasound. Science. 1955;122:517–8.
Hynynen K, Lulu BA. Hyperthermia in cancer treatment. Invest Radiol. 1990;25:824–34.
Sanghvi NT, Foster RS, Bihrle R, et al. Noninvasive surgery of prostate tissue by high intensity focused ultrasound: an updated report. Eur J Ultrasound. 1999;9:19–29.
Gelet A, Chapelon JY, Bouvier R, et al. Transrectal high-intensity focused ultrasound: minimally invasive therapy of localized prostate cancer. J Endourol. 2000;14:519–28.
Visioli AG, Rivens IH, ter Haar GR, et al. Preliminary results of a phase I dose escalation clinical trial using focused ultrasound in the treatment of localised tumours. Eur J Ultrasound. 1999;9:11–8.
ter Haar G. Biological effects of ultrasound in clinical applications. In: Suslick K, editor. Ultrasound: its chemical, physical, and biological effects. New York: VCH; 1988. p. 305–20.
Yuan C, Yang L, Yao C. Observation of high intensity focused ultrasound treating 40 cases of pancreatic cancer [in Chinese]. Chin J Clin Hepatol. 2003;19:145–6.
Sofuni A, Itoi T, Itokawa F, et al. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J Gastroenterol. 2008;14:7183–91.
He SX, Wang GM. The noninvasive treatment of 251 cases of advanced pancreatic cancer with focused ultrasound surgery. In: Andrew MA, Crum LA, Vaezy S, editors. Proceedings from the 2nd International Symposium on Therapeutic Ultrasound. Seattle: University of Washington; 2002. p. 51–6.
Gu Y, Wang G, Xia H, et al. Application of high intensity focused ultrasound in treating 45 cases of carcinoma of the pancreas [in Chinese]. Fudan Univ J Med Sci. 2005;31:135–41.
Wang RS, Mu QX, Liu LX, Shu YQ. A clinical study of thermotherapy of HIFU in combination with chemotherapy on treating advanced pancreatic cancer [in Chinese]. Acta Nanjing Med Univ. 2003;23:460–3.
Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound in treating patients with advanced pancreatic carcinoma [in Chinese]. Chin J Gen Surg. 2002;17:654–5.
Wu F, Wang ZB, Zhu H, et al. Feasibility of US guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–40.
Xie DR, Chen D, Teng H. A multicenter nonrandomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma [in Chinese]. Chin J Clin Oncol. 2003;30:630–4.
Xiong LL, He CJ, Yao SS, et al. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound [in Chinese]. Chin J Gen Surg. 2005;16:345–7.
Xu YQ, Wang GM, Gu YZ, Zhang HF. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma [in Chinese]. Clin Med J China. 2003;10:322–3.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sofuni, A., Moriyasu, F., Sano, T. et al. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci 18, 295–303 (2011). https://doi.org/10.1007/s00534-010-0355-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-010-0355-4