Abstract
Liver function reserve estimation is important for selecting the appropriate patients for hepatectomy or ablation of tumors. Many liver function tests have been devised, but the indocyanine green (ICG) clearance test remains the most popular for its simplicity and perhaps accuracy. Compared with the Child–Pugh classification, the ICG retention value at 15 min (ICGR-15) after intravenous injection provides more information. Though a significant difference in ICGR-15 has been observed between patients with Child–Pugh A and B liver function, the hospital mortality rates following partial hepatectomy are not significantly different between the two groups. Yet, ICGR-15 values can differentiate patients with or without hospital mortality. The cutoff values of ICGR-15 for a safe major and minor hepatectomy are 14 and 22%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is one of the largest organs in the body. Liver failure leads to impaired protein synthesis, accumulation of metabolic waste, clotting deficiency, and a predisposition to infection. Resection of a normal liver up to 75% of the total volume is not fatal because the liver regenerates rapidly [1, 2]. However, in most of the situations that necessitate partial hepatectomy, i.e., liver tumor, the liver is abnormal, its functional reserve is reduced, and regeneration is slow or absent. Resection of abnormal liver is therefore risky. In the past few decades, the results of partial hepatectomy of abnormal liver have improved [3, 4]. Better selection of patients based on liver function status could have been the most important contributory factor to the success. However, an accurate, yet practical and cost-effective liver function measurement has not been clearly defined. In this review, our views and experience on liver function reserve estimation are presented.
Causes of liver injury
The liver can be destroyed by disease, drugs, or chemicals. Excessive inflammatory reaction to injury ultimately leads to fibrosis and cirrhosis. In such situations, the liver function is impaired because hepatocyte perfusion is reduced by the increased amount of extracellular matrix and decreased vascular space. Chemotherapy-induced liver toxicity is now recognized as an important cause of liver failure after hepatectomy in patients with colorectal cancer metastasis [5]. Liver tumor itself seldom induces liver damage unless the tumors are numerous or occlude the inflow and outflow vessels of the liver or bile duct.
Routine laboratory tests
Liver function can be assessed readily by routine laboratory tests. Serum albumin and bilirubin levels are surrogate markers of synthetic and excretory functions, respectively. Platelet and white cell counts are reflective of portal hypertension. Measurement of the blood ammonia level is rarely performed, except in cases of hepatic encephalopathy. Raised serum alanine aminotransferase and aspartate aminotransferase levels are indicative of ongoing hepatocyte destruction rather than actual liver function. Instead, the prothrombin time or the international normalized ratio is a real-time reflection of liver function. Hypoglycemia rarely occurs unless the patient is in fulminant hepatic failure. Some of these tests have been incorporated in the Child–Pugh classification of liver function (A, B, C) and the “degree of liver damage” scoring system (A, B, C) designed by the Liver Cancer Study Group of Japan [6]. In both systems, only patients with “A” liver function are considered suitable for partial hepatectomy, while patients with “B” liver function are borderline cases. An intrinsic problem of both systems is that subjective parameters, such as ascites and hepatic encephalopathy, are included and these render the assessment less accurate. To eliminate the subjective parameters, the aspartate aminotransferase/platelet count ratio index, which is a biochemical surrogate for histological fibrogenesis in cirrhosis, may be an alternative [7]. It was shown to be predictive of posthepatectomy liver failure. Another approach based on readily available laboratory tests is the model for end-stage liver disease (MELD) score [8], which incorporates the international normalized ratio, serum bilirubin level, and serum creatinine level in the calculation. The disadvantage of employing the MELD score is that many hepatectomy patients have normal serum creatinine concentrations, resulting in most of the patients having a low MELD score. Thus, the range of MELD scores that has been used for the prediction of posthepatectomy survival is quite narrow [9]. Nevertheless, if patients have underlying or hidden renal disease, they run a higher chance of dying from the operation. Indeed, impaired renal function has been identified to be an important prognostic factor after hepatectomy [10]. Patients with impaired renal function should not be subject to hepatectomy unless perioperative hemodialysis is planned.
Indocyanine green clearance test
The indocyanine green (ICG) clearance test is the most commonly used liver function test in Asia [11] and is increasingly used in other parts of the world. ICG is a harmless dye, but occasionally an allergic response to it has been observed after intravenous administration. After intravenous administration, it binds completely to albumin and β-lipoprotein and is exclusively removed by the liver and excreted unchanged in bile without any entero-hepatic circulation [12]. It is mainly a measurement of the liver blood flow and a reflection of intrahepatic portovenous shunt and sinusoidal capillarization [13]. The ICG retention value at 15 min (ICGR-15) after intravenous injection is about 10% in normal persons. The ICG clearance test result has been used singly or in combination with other parameters in recommendations for the extent of hepatectomy. When the test is used alone, the cutoff value of ICGR-15 after intravenous injection allowing a safe major hepatectomy is 14% [14]. With experience, the cutoff value for a safe major hepatectomy can be pushed to 17%, but only in relatively young patients and those with adequate remnant liver volume [15]. Limited resection (without sacrifice of nontumorous liver) may be allowed for higher ICGR-15 values. The upper limit was set at 40% by Makuuchi et al. [16]. Combined with the serum bilirubin level and findings of ascites, the ICGR-15 has been incorporated in a decision tree for determination of the extent of hepatectomy. An almost zero percent hospital mortality rate has been achieved in a large series of patients selected for hepatectomy based on the decision tree [17].
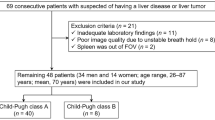
Compared with the Child–Pugh classification, the ICG clearance test appears to provide more information (Fig. 1). Although a significant difference in median ICGR-15 values has been observed between Child–Pugh A and B hepatocellular carcinoma patients, a wide range of ICGR-15 values was seen in both groups of patients. Taking ICGR-15 as a true reflection of liver function, the patients classified as Child–Pugh A or B were actually quite heterogeneous in terms of their liver functions. It is therefore not unexpected that the difference in the hospital mortality rates between Child–Pugh A and B patients undergoing hepatectomy was not significant (4.3 vs. 7.7%, P = 0.242). However, based on ICGR-15 cutoff values of 10, 14, and 17%, significantly higher hospital mortality rates were observed in patients with higher ICGR-15 values (Child–Pugh A and B patients, 2.4 vs. 5.8%, P = 0.007; 3.5 vs. 6.3%, P = 0.043; and 3.6 vs. 7.7%, P = 0.01, respectively), indicating that ICGR-15 values are more predictive of the outcome than the Child–Pugh classification in hepatocellular carcinoma patients undergoing hepatectomy. Based on the data of our patients, the ICGR-15 cutoff value for a safe major hepatectomy is 14% and that for a minor hepatectomy is 22%. An added benefit of the ICG clearance test is that 64% of Child–Pugh class B patients had an ICGR-15 of <22% (Fig. 1). If such patients had been evaluated by the Child–Pugh classification alone, they would not have been considered for hepatectomy.
While clinically useful, the ICG retention value should be interpreted with caution. Falsely high retention values are seen in patients with portal vein obstruction, bile duct obstruction, and Gilbert syndrome. Before a patient is declined for hepatectomy because of a high ICG retention value, careful evaluation of imaging and history is necessary. Surgeons should look for possibilities of improving the ICG retention value, thus maximizing the patient’s chance of receiving meaningful treatments. Treatments that may improve the ICG clearance include antiviral therapy, biliary decompression, and portal vein tumor thrombus extraction or portal vein resection. The limitation of the ICG clearance test is that it is not routinely available in many hospitals. To replace laborious measurement and repeated blood-taking, infrared digital measurement based on pulse spectrophotometry is now used at the bedside and has proven to be convenient and accurate in predicting postoperative liver failure [18–20]. Excellent correlation has also been shown between the standard and pulse spectrophotometry methods [21]. Measuring the elimination constant of ICG by pulse spectrophotometry may emerge as the most convenient bedside dynamic liver function test.
Other liver function tests
Many quantitative estimations of liver function based on the principle of clearance of substrate by the liver have been developed. The substrates include lidocaine [22], galactose [23], aminopyrine [24], amino acid [12], and methacetin [25]. Tests based on these substrates have not been shown to be superior to the ICG clearance test in the prediction of posthepatectomy liver failure or complications. There are also tests that are based on the energy production of the liver (arterial ketone body ratio; AKBR) [26, 27] and the number of receptors for asialoglycoprotein (ASGP-R; technetium-99m-galactosyl human serum albumin; 99mTc-GSA scan) [28, 29]. The AKBR test requires sampling from arterial blood and is therefore inconvenient. The 99mTc-GSA scan may accurately reflect functional liver mass, since ASGP-R is absent from fibrous tissue in chronic liver disease [29]. A good correlation of the 99mTc-GSA scan result with ICGR-15 has been shown. However, the 99mTc-GSA scan is expensive and therefore lacks popularity.
Clinical implications of liver function tests
The major practical value of liver function tests is the estimation of operative risk from partial hepatectomy [12] or an ablation procedure [30]. Patients with poor liver function reserve are more likely to succumb because of loss of functional liver mass and poor tolerability to general anesthesia, the stress of the operation, and bleeding. Estimation of the adequacy of the residual liver volume is therefore an important parameter, in addition to measurement of liver function, in the overall preoperative evaluation. Both the quantity and the quality of the liver remnant are important considerations. Measurement of the remnant liver volume can be accurately performed by computed tomography or magnetic resonance imaging. Assessment of the adequacy of liver volume for postoperative survival can be based on the estimated standard liver volume of an individual [31] or the amount of nontumorous liver on computed tomography volumetry. If the latter is difficult to estimate because of an indistinct boundary between the tumor and the normal liver, then the estimated standard liver volume would be a better denominator for the calculation of the required remnant liver volume. For a normal liver, a residual liver volume of 25% of the estimated standard liver volume would be sufficient for postoperative survival [1]. For an abnormal liver, a larger volume (35–40%) is required [32].
To assess the quality of the remnant liver, liver biopsy has been advocated. This invasive procedure may be replaced by transient elastography [33], but the prediction of postoperative outcome by the findings of transient elastography has not been established.
The reason that researchers continue to develop new liver function tests even up to this day is because none of the liver function tests currently available are absolutely correct in predicting the postoperative outcome. The deficiency does not lie in the tests themselves, but in the perception of many surgeons that the remnant liver can tolerate all intraoperative insults and the patient can survive if the liver function is normal preoperatively. In fact, postoperative liver function deterioration is due to many inter-related intraoperative and postoperative events. Technical errors, e.g., massive bleeding, prolonged inflow occlusion, prolonged outflow occlusion, residual necrotic liver tissue, and biliary leakage, are conducive of postoperative liver failure even if the initial liver function was normal [34] (Fig. 2). It is not surprising, therefore, that immediate postoperative liver function assessments are highly predictive of liver failure [35–37], but such postoperative liver function assessments are not relevant for selecting the appropriate patients for hepatectomy. To ensure uniform success, meticulous attention to detail in all surgical endeavors is of paramount importance. The estimation of liver function reserve is only part of the overall management and only part of the contribution to the selection of appropriate patients for hepatectomy.
References
Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–30.
Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ, Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–96.
Fan ST. Hepatectomy for hepatocellular carcinoma: towards zero hospital deaths. Ann Surg. 1999;229:322–30.
Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–92.
Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–44.
Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd English ed. Tokyo: Kanehara; 2003. p. 13–14.
Ichikama T, Uenishi T, Takemura S, Oba K, Ogawa M, Kodai S, et al. A simple, noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:42–8.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70.
Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, et al. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch Surg. 2009;144:57–63.
Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy. Ann Surg. 2004;240:698–708.
Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–9.
Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961;21:43–57.
Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009;13:374–85.
Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203.
Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012–7.
Makuuchi M, Hasegawa H, Yamazaki S. Indication for hepatectomy in patients with hepatocellular carcinoma and cirrhosis (in Japanese). Shindan Chiryo. 1986;74:1225–30.
Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22.
Ohwada S, Kawate S, Hamada K, Yamada T, Sunose Y, Tsutsumi H, et al. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg. 2006;93:339–46.
Purcell R, Kruger P, Jones M. Indocyanine green elimination: a comparison of the LiMON and serial blood sampling methods. ANZ J Surg. 2006;76(1–2):75–7.
de Liguori Carino N, O’Reilly DA, Dajani K, Ghaneh P, Poston GJ, Wu AV. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol. 2009;35(9):957–62.
Hsieh CB, Chen CJ, Chen TW, Yu JC, Shen KL, Chang TM, et al. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J Gastroenterol. 2004;10:2394–6.
Oellerich M, Armstrong VW. The MEGX test: a tool for the real-time assessment of hepatic function. Ther Drug Monit. 2001;23:81–92.
Zoedler T, Ebener C, Becker H, Roeher HD. Evaluation of liver function tests to predict operative risk in liver surgery. HPB Surg. 1995;9:13–8.
Fan ST, Wang QS, Lo CM, Tam Yu KW, Lai EC, Wong J. Evaluation of indocyanine green retention and aminopyrine breath tests in patients with malignant biliary obstruction. Aust N Z J Surg. 1994;64:759–62.
Shirin H, Aeed H, Shalev T, Sorin V, Stavinski S, Shahmurov M, et al. Utility of a 13C-methacetin breath test in evaluating hepatic injury in rats. J Gastroenterol Hepatol. 2008;23:1762–8.
Kiuchi T, Ozawa K, Yamamoto Y, Takayasu T, Maki A, Shimahara Y, et al. Changes in arterial ketone body ratio in the phase immediately after hepatectomy. Prognostic implications. Arch Surg. 1990;125:655–9.
Hanazaki K, Wakabayashi M, Sodeyama H, Makiuchi A, Igarashi J, Yokoyama S, et al. Arterial ketone body ratio does not correlate with ischemic changes during major hepatectomy. Hepatogastroenterology. 1998;45:145–9.
Hwang EH, Taki J, Shuke N, Nakajima K, Kinuya S, Konishi S, et al. Preoperative assessment of residual hepatic functional reserve using 99mTc-DTPA-galactosyl-human serum albumin dynamic SPECT. J Nucl Med. 1999;40:1644–51.
Kwon AH, Matsui Y, Kaibori M, Ha-Kawa SK. Preoperative regional maximal removal rate of technetium-99m-galactosyl human serum albumin (GSA-Rmax) is useful for judging the safety of hepatic resection. Surgery. 2006;140:379–86.
Cheung TT, Ng KK, Poon RT, Fan ST. Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(5):655–60.
Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217–22.
Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–81.
Shiha G, Sarin SK, Ibrahim AE, Omata M, Kumar A, Lesmana LA, et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int. 2009;3:323–33.
Fan ST, Lo CM, Liu CL. Major hepatic resection for primary and metastatic tumors. In: Fischer JE, Bland KI, Callery MP, LoGerfo FW, Clagett GP, Seeger JM, et al., editors. Mastery of surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 1076–91.
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–8.
Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–25.
Cucchetti A, Ercolani G, Cescon M, Ravaioli M, Zanello M, Del Gaudio M, et al. Recovery from liver failure after hepatectomy for hepatocellular carcinoma in cirrhosis: meaning of the model for end-stage liver disease. J Am Coll Surg. 2006;203:670–6.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fan, S.T. Liver functional reserve estimation: state of the art and relevance for local treatments. J Hepatobiliary Pancreat Sci 17, 380–384 (2010). https://doi.org/10.1007/s00534-009-0229-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0229-9