Abstract
Background/purpose
In patients with hilar biliary malignancies, preservation of the middle hepatic artery (MHA, segment IV artery) where it runs close to the tumor in the hepatic hilum may lead to resection with positive margins. This retrospective study assessed the safety of combined resection of the MHA with right hemihepatectomy, caudate lobectomy, and bile duct resection for hilar biliary malignancies.
Methods
Of 61 patients with hilar biliary malignancies who underwent right hemihepatectomy, we classified the branching patterns of the MHA according to the origins and courses in the hilum. The MHA was resected without reconstruction in 16 patients in whom the artery ran close to the tumor. We compared the perioperative outcomes in these patients with those of patients who did not undergo resection of the artery.
Results
Anatomically, the MHA ran on the right side of the umbilical portion of the portal vein in 40 (66%) patients. Perioperative data for the patients who underwent combined resection were similar to those in whom the MAH was preserved. There were no postoperative complications that could be directly related to the arterial resection.
Conclusions
Combined resection of the MHA during right hemihepatectomy for hilar biliary malignancies has a safe perioperative course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hilar biliary malignancies, including hilar cholangiocarcinoma and gallbladder cancer, often invade the hepatoduodenal ligament. The right hepatic artery and its nerve plexus, which passes on the right behind the hilar bile duct, typically necessitate resection because of the potential for perineural infiltration of the tumor [1, 2]. In anatomical studies, the middle hepatic artery (MHA, segment IV artery) arises from the right hepatic artery in approximately one-fourth of cases [3, 4]; when it does, the MHA and/or its plexus can be infiltrated by tumor. When the MHA originates from the left hepatic artery, it often runs on the right side of the umbilical portion of the portal vein (UP), although there has been no systematic investigation to determine the frequency with which the MHA adopts this course. In such both cases, preservation of the MHA during right hemihepatectomy might result in a positive resection margin, because the artery runs in close proximity to the tumor. Preservation of the MHA is the standard technique in right hemihepatectomy for hilar biliary malignancy. Although hypoxic hepatic parenchymal injury does not occur if portal flow is maintained [6–8], interruption of the arterial flow can cause serious postoperative complications related to biliary ischemia, including disruption of the bilioenteric anastomosis and liver abscess [5]. For a series of patients undergoing living-related liver transplantation success was reported for left lobe grafts without reconstruction of the MHA [9]. Pulsatile back-flow bleeding from the arterial stump of the medial section after reconstruction the lateral section artery was observed in that series, implying the existence of collateral arterial communications between the medial and the lateral sections.
In right hemihepatectomy, arterial communications may play a crucial role in the prevention of the complications related to ischemia of the hepatic ducts because of arterial interruption. The results of middle hepatic arterial resection during right hemihepatectomy for hilar biliary malignancy, however, have not been reported. In this retrospective study, we investigated the anatomical variations of the MHA to stratify the risk of cancer invasion into the artery. We also assessed the safety of resection of the MHA combined with right hepatectomy, caudate lobectomy, and bile duct resection.
Patients and methods
From January 1999 to March 2007, 61 patients with hilar cholangiocarcinoma or gallbladder cancer underwent combined right hemihepatectomy, caudate lobectomy, and bile duct resection with curative intent. Anatomic variations of the MHA, confirmed by preoperative imaging and intraoperative findings, were classified according to their relationship to the UP.
In 16 of the 61 patients, the MHA was resected concomitantly (resection group), while the MHA was preserved in the remaining 45 patients (preservation group). Fifteen patients in the resection group underwent combined resection to ensure negative surgical margins, because preoperative imaging or intraoperative findings indicated that the tumor was located in close proximity to the MHA. In the remaining patient in the resection group, intraoperative injury of the MHA necessitated combined resection. We compared the patients’ characteristics between the groups (Table 1). The median age of the patients in the resection group was significantly older than that in the preservation group. The proportion of female patients was much higher for the resection group than for the preservation group. In the resection group the primary cancers were hilar cholangiocarcinoma and gallbladder carcinoma, each in eight patients. In the preservation group the primary cancers were hilar cholangiocarcinoma and gallbladder carcinoma in 32 and 13 patients, respectively. Two patients in the resection group and 11 patients in the preservation group underwent combined pancreatoduodenectomy and right hemihepatectomy (hepatopancreato-duodenectomy). The portal bifurcation was reconstructed in 12 and 36 patients in the resection and preservation groups, respectively. Preoperative biliary decompression and portal embolization was performed similarly in both the preservation and resection groups. Preoperative hepatic functional reserve, evaluated by an indocyanine green (ICG) clearance test, did not differ between the two groups.
To assess the safety of the procedure, we compared data, including postoperative hepatic function and perioperative outcomes, between the two groups. Any postoperative complications which prolonged the hospital stay were defined as morbidity.
Operative procedures
The first step of the procedure of dissecting the hepatoduodenal ligament was exposure of the proper and left hepatic arteries. The left hepatic artery was fully mobilized toward the side of the left portal vein. Only the proximal part of the right hepatic artery was dissected to prevent the tumor from being exposed. In the resection group, the MHA was not exposed when it arose from the right hepatic artery. When it originated from the left or proper hepatic arteries, the MHA was divided immediately after branching. The MHA was divided distally at the same level as the excision of the left hepatic duct adjacent to the umbilical portion. The detailed procedures for skeletonizing the hepatoduodenal ligament, lymphadenectomy, hepatectomy, and division of the left hepatic duct, and its reconstruction, have been described previously [10]. To reach the hepatic duct adjacent to the umbilical portion during the hepatic dissection, only a small part of segment 4 was concomitantly resected in every case. Portal reconstruction was performed prior to dissection of the hepatic parenchyma [10].
Statistical analysis
Data from the groups were compared by use of the Mann–Whitney U test, the chi-squared test, or Fisher’s exact test. P values less than 0.05 were considered to be statistically significant.
Results
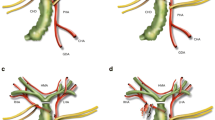
Anatomic variations of the MHA in this series were classified into five types (Figs. 1, 2). In types R-1, R-2, and R-3, the MHA ran on the right side of the umbilical potion, arising from the right hepatic artery (R-1) in 19 patients (31%), the left hepatic artery (R-2) in 19 patients (31%), and the proper hepatic artery (R-3) in two patients (3.5%). In the R-3 variant, the MHA is the single artery continuation of the proper hepatic artery, as the right hepatic artery originated from the superior mesenteric artery and the left hepatic artery arose from the left gastric artery. Two patients (3.5%) were classified as type R-4, in which the left hepatic artery ran on the right side of the UP, giving rise to the lateral section arteries proximally and the MHA (artery to the medial section) distally. Nineteen patients (31%) were classified as type L, in which the MHA originated from the left hepatic artery to run behind the UP. Patients who underwent combined resection were classified as having MHA variants of types R-1 (ten patients), R-2 (four patients), R-3 (one patient), and L (one patient). Intraoperative injury was the reason for concomitant resection of the MHA in the patient with anatomy classified as type L.
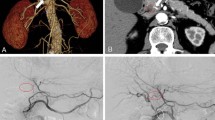
Computed tomography (arterial phase) findings of the middle hepatic artery (MHA). The anatomical variations of the MHA were classified according to their relationship to the umbilical portion of the portal vein (UP). When running on the right side of the UP, MHA variants were classified into three types, as arising from the right hepatic artery (R-1), from the left hepatic artery (R-2), or from the proper hepatic artery (R-3) as a single artery, when the right hepatic artery branched from the superior mesenteric artery and the left hepatic artery arose from the left gastric artery. In type R4, the left hepatic artery branched off from the lateral section arteries, and the MHA coursed along the right side of the UP. In type L, the MHA originated from the left hepatic artery to run behind the UP. M middle hepatic artery. R right hepatic artery. L left hepatic artery. LG left gastric artery. C catheter for biliary decompression
Our comparison of surgical outcomes and postoperative hepatic function did not reveal any differences between the groups. No pathological residual tumor was observed in the resection group. Two of the patients in the preservation group had pathologically positive bile duct margins because of broad intraepithelial spread of the tumor (Table 2). Morbidity was similar in the resection and preservation groups. Postoperative in-hospital mortality did not differ between the groups. We did not encounter any complications that could be directly related to MHA resection in the resection group. Hepatic abscess formation occurred in one patient in the resection group; this development was determined to be unrelated to bile duct ischemia from arterial resection, because multiple abscesses developed in both the lateral and medial sections. One patient in the resection group who experienced bile leakage from the cholangiojejunostomy was treated conservatively with an anastomotic stent for external biliary drainage and a drainage tube that had been placed prophylactically near the anastomosis intraoperatively. One patient in the resection group suffered from postoperative septic shock because of severe enteritis with methicillin-resistant Staphylococcus aureus infection and later died from hepatic failure. The postoperative hospital stay duration was similar between the two groups (Table 3).
Discussion
The hepatic dual blood supply and extensive vascular collateral pathways are thought to protect the liver from ischemic insult [11]. Normal flow of the portal vein is thought to deliver sufficient oxygen to the liver; thus, hepatic artery ligation has been performed as a hemostatic procedure for severe hepatic injury [12]. In patients with extrahepatic bilioenteric anastomosis, however, interruption of hepatic arterial flow can often lead to anastomotic insufficiency and/or liver abscess [5], even when normal portal flow is maintained [6, 8]. Similarly, following hepatic lobectomy for biliary malignancy, a lack of arterial flow to the remnant hepatic lobe can lead to a distressing postoperative course complicated by dehiscence of the intrahepatic cholangiojejunostomy, liver abscess, and hepatic failure [7, 13]. Arterial thrombosis after orthotopic liver transplantation can lead to necrosis of the bile duct [14] and anastomotic and nonanastomotic intrahepatic bile duct strictures, which are assumed to result from ischemia [15, 16]. Thus, maintenance of arterial blood flow to the biliary system is important to prevent significant postoperative complications.
Ikegami et al. [9]. described ten cases of successful orthotopic living-related liver transplantation using a left lobe graft without reconstruction of the MHA (artery to segment IV). In this study, pulsatile back flow from the stump of the MHA was observed after reconstructing the artery to the lateral section in ten out of twelve cases. This result suggests that connections between the MHA and the lateral section arteries preexist within the liver in most cases. Patients in this study who underwent concomitant resection of the MHA with right hemihepatectomy did not experience negative postoperative outcomes that could be directly related to ischemic injury, for example hepatic infarction, liver abscess, or anastomotic leakage. Postoperative hepatic function in patients who underwent MHA resection was similar to that seen in patients with preservation of the artery. These data indicate that blood flow to the biliary system and hepatic parenchyma of the medial section was maintained after interrupting flow of the MHA.
Anatomic study of the microcirculation of the liver revealed that the intrahepatic bile ducts are fed by a dense surrounding vascular plexus, dubbed the peribiliary vascular plexus, arising from the hepatic artery [17]. This vascular plexus is included in the plate system in the hepatic hilar area and in each Glissonian pedicle in the peripheral region, which is contiguous with the plate system [18]. Therefore, the arterial network surrounding the bile duct continues undivided as far as the Glissonian pedicle or the plate system. Miyazaki et al. [13]. reported cases of bile duct cancer in which only the unilateral lobar hepatic artery was reconstructed; arterial supply to the contralateral lobe was facilitated by the preserved plate system around the confluence of the hepatic duct. These arteries were described as interlobar arteries in the literature. During right hemihepatectomy, on the right side of the umbilical portion where the left hepatic duct is usually divided, both bile ducts from the medial and lateral sections are still included in the umbilical plate and are not separated into each Glissonian pedicle [18]. Thus, the peribiliary plexuses of the bile ducts from the medial and lateral sections may retain their connections through the plate system, compensating for any loss of arterial blood supply to the medial branch of the bile duct. Another possibility is that compensatory arterial blood supply to the area fed by the interrupted artery may derive from intrahepatic interconnecting arterial pathways [19]; the clinical implications of such pathways have not yet been demonstrated. Also, vessels connecting the hepatic artery and the portal system, identified by electron microscopic studies [20], are also a possible source of blood supply. The efficacy of such connections has been clinically demonstrated by the arterio-portal shunting used in cases of complete dearterialization of the hepatoduodenal ligament [21]. The connection between the arterial and portal systems may play an important role in amelioration of the severe ischemic condition following complete loss of the arterial circulation.
Anatomically, the MHA coursed along the right side of the UP in 40 of the 61 (66%) patients examined in this study. In biliary malignancies, tumor extension occurs along the perineural spaces of the autonomic nerve plexuses around the hepatic arteries [1]. As perineural invasion of the tumor cannot be diagnosed accurately by preoperative radiological examination, en bloc and concomitant resection of arteries in close proximity to the tumor should be considered to obtain negative margins. Therefore, patients in whom the MHA runs along the right side of the UP should be candidates for combined resection of the artery with right hemihepatectomy. MHA resection would not be indicated for patients with R-4 and L-type anatomic variants of the MHA, because the MHA was not recognized in the hilar region. Surgeons should pay attention to the location of the tumor, mode of tumor spread, and route of the MHA when considering performing combined resection of the artery. It is important to note that the left hepatic artery can rarely provide tributaries to the medial and lateral sections when the MHA runs along the right side of the UP (R-4 type in our classification). Without this understanding, arteries to the lateral section may be mistakenly divided during the intended resection of the MHA.
In conclusion, combined resection of the MHA during right hemihepatectomy for hilar biliary malignancy is safe from the viewpoint of the perioperative course. This procedure may prevent failure to remove residual cancer in anatomically appropriate patients with hilar biliary malignancies with perineural invasion.
References
Yamaguchi R, Nagino M, Oda K, Kamiya J, Uesaka K, Nimura Y. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br J Surg. 2002;89:1130–6.
Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101.
Skandalakis JE, Skandalakis LJ, Skandalakis PN, Mirilas P. Hepatic surgical anatomy. Surg Clin North Am. 2004;84:413–35.
Onishi H, Kawarada Y, Das BC, Nakano K, Gadzijev EM, Ravnik D, et al. Surgical anatomy of the medial segment (S4) of the liver with special reference to bile ducts and vessels. Hepatogastroenterology. 2000;47:143–50.
Majno PE, Prêtre R, Mentha G, Morel P. Operative injury to the hepatic artery. Consequences of a biliary-enteric anastomosis and principles for rational management. Arch Surg. 1996;131:211–5.
Gupta N, Solomon H, Fairchild R, Kaminski DL. Management and outcome of patients with combined bile duct and hepatic artery injuries. Arch Surg. 1998;133:176–81.
Tanaka K, Nishimura A, Hombo K, Furoi A, Ikoma A, Yamauchi T, et al. The development of a pyogenic liver abscess following radical resection of cholangiocellular carcinoma with ligation of the right hepatic artery: report of a case. Surg Today. 1994;24:659–62.
Smith GS, Birnbaum BA, Jacobs JE. Hepatic infarction secondary to arterial insufficiency in native livers: CT findings in 10 patients. Radiology. 1998;208:223–9.
Ikegami T, Kawasaki S, Matsunami H, Hashikura Y, Nakazawa Y, Miyagawa S, et al. Should all hepatic arterial branches be reconstructed in living-related liver transplantation? Surgery. 1996;119:431–6.
Kondo S, Katoh H, Hirano S, Ambo Y, Tanaka E, Okushiba S. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694–7.
Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974;290:993–6.
Parks RW, Chrysos E, Diamond T. Management of liver trauma. Br J Surg. 1999;86:1121–35.
Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Yoshidome H, et al. Unilateral hepatic artery reconstruction is unnecessary in biliary tract carcinomas involving lobar hepatic artery: implications of interlobar hepatic artery and its preservation. Hepatogastroenterology. 2000;47:1526–30.
Stratta RJ, Wood RP, Langnas AN, Hollins RR, Bruder KJ, Donovan JP, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery. 1989;106:675–83.
Colonna JO 2nd, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, et al. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344–50.
Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517–24.
Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–70.
Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg. 2000;7:580–6.
Arnold MM, Kreel L, Lo YF, Law H. Are the hepatic arteries “end arteries”? Invest Radiol. 1991;26:337–42.
Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8:139–49.
Kondo S, Hirano S, Ambo Y, Tanaka E, Kubota T, Katoh H. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg. 2004;91:248–51.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hirano, S., Kondo, S., Tanaka, E. et al. Safety of combined resection of the middle hepatic artery in right hemihepatectomy for hilar biliary malignancy. J Hepatobiliary Pancreat Surg 16, 796–801 (2009). https://doi.org/10.1007/s00534-009-0107-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0107-5