Abstract
Pancreaticobiliary maljunction (PBM) is associated with the occurrence of biliary cancer due to pancreatobiliary reflux. We present a case of simultaneous double cancer of the gallbladder and bile duct. A 77-year-old woman who had jaundice, intra- and extra-hepatic biliary ductal dilatation and a space-occupying lesion in the gallbladder and lower bile duct underwent pancreatoduodenectomy. The gallbladder cancer showed papillary carcinoma without mutation of the K-ras gene and with p53 non-sense mutation of CCA (Pro) to CA (Stop) on codon 301 in exon 8. The bile duct cancer revealed a well-differentiated adenocarcinoma without mutation of the K-ras gene and with p53 miss-sense mutation of GTG (Val) to GAG (Glu) on codon 272 in exon 8. There were no mutations of either the K-ras or p53 gene in non-cancerous epithelia. In contrast, only the mucosa of the common channel had p53 protein accumulation and high cell proliferation activity. Therefore, the genetic pathway might be the same in both the gallbladder and bile duct cancer, and a high potential for carcinogenesis might be present in the epithelium of the common channel in patients with PBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
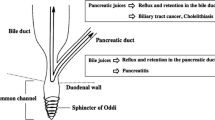
Pancreaticobiliary maljunction (PBM) is a congenital anomaly defined as a union of the pancreatic and biliary ducts outside of the duodenal wall [1–3]. PBM develops because of an arrest in the migration of the choledochopancreatic junction into the duodenal wall before the eighth week of gestation and shows a long common channel with the absence of a septum between the bile duct and the pancreatic duct [1–3]. The high risk of PBM for biliary tract cancer has been reported [2]. Pancreatic juice refluxes into the biliary tract and pools in the gallbladder and bile duct, resulting in activation of pancreatic enzymes, and is associated with the pathogenesis of biliary cancer. Therefore, the atypical epithelium frequently found in the common channel is the most important site of pathogenesis of cancer of the papilla of Vater [4]. Genetic analysis shows multiple genetic mutations, among which K-ras gene activation and the p53 tumor suppressor gene inactivation in the mucosa of the gallbladder and bile duct are recognized as the most important keys for carcinogenesis in PBM [5–7].
We performed pancreatoduodenectomy for a PBM patient with simultaneous double cancer of the gallbladder and lower bile duct. To clarify the relationship between pathologic and genetic changes, we examined the p53 and the K-ras gene mutation and p53 protein overexpression in both carcinomas, the epithelium of the bile duct, pancreatic duct, common channel and the mucosa adjacent to the papilla of Vater.
Case report
A 77-year-old woman was admitted with jaundice on 20 March 2006. Abdominal computerized tomography (CT) and ultrasonography (US) showed intra- and extra-hepatic biliary duct dilatation and space occupying lesions in the gallbladder (Fig. 1a) and lower bile duct, respectively. Endoscopic retrograde cholangiopancreatography (ERCP) revealed an incomplete obstruction in the lower portion of the common bile duct. The cholangiopancreatography showed that the main pancreatic duct joined the bile duct 30 mm above the papilla of Vater and the main pancreatic duct joined the common bile duct (Type 1, or P–C union) [8] (Fig. 1b). We performed retrograde endoscopical biliary drainage and biopsies from a lower bile duct. Adenocarcinoma was detected in the biopsy specimens. Tumor markers, including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA) were within normal ranges. We diagnosed synchronous double cancers associated with PBM and performed pancreatoduodenectomy with lymph node dissection. Histological specimens revealed that the double cancers were not connected (Fig. 1c, d). The gallbladder lesion was papillary carcinoma (Fig. 1e) and that of the common bile duct was well differentiated tubular adenocarcinoma (Fig. 1f). We dissected 5 mm2 fresh specimens from areas (A)–(F) of the resected specimens as shown in Fig. 2a. The specimens were divided into two parts; one was immediately frozen in liquid nitrogen and stored at −80°C for later DNA extraction and the other was chilled and fixed with Tissue-Tek freezing medium (Sakura Finetechnical Co., Ltd, Tokyo, Japan) for H&E and p53 immunohistochemical staining as described below. The remaining surgical specimens fixed in 10% formalin and embedded in paraffin for routine histological diagnosis. DNA was extracted from each frozen sample by the phenol–chloroform method and amplified using four pairs of forward and reverse primers labeled at 5′ end with Cy5 (Amersham Pharmacia Biotech, Piscataway, NJ, USA) for p53 gene analysis: exon 5: 5′-TTCCTCTTCCTACAGTACTCC and 5′-GCCCCAGCTGCTCACCATCGC, exon 6: 5′-CACTGATTGCTCTTAGGTCTG and 5′-AGTTGCAAACCAGACCTCAGG, exon 7: 5′-CCAAGGCGCACTGGCCTCATC and 5′-TCAGCGGCAAGCAGAGGCTGG, exon 8: 5′-CCTATCCTGAGTAGTGGTAAT and 5′-GTCCTGCTTGCTTACCTCGCT and TM High Fidelity PCR System (Roche Ltd, Basel, Switzerland). Electrophoresis of PCR products were done at 1,200 V for 5 h with ALF Express (Amersham Pharmacia Biotech, Piscataway, NJ, USA). A single-strand conformation polymorphism (SSCP) analysis was performed on analytic software Allele Link (Amersham Pharmacia Biotech). For extra peak of SSCP analysis, sequence was examined with a purified PCR product with a high pure PCR product purification kit (Roche), thermo sequenase fluorescent labelled primer cycle sequencing kit with 7-deaza-dGTP (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and analyzed by Analytic Software Evaluation (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Cancer of the gallbladder and the bile duct. a The elevated region (arrow) was shown in the lumen of gallbladder on CT. b Cholangiopancreatogram demonstrated a long common channel in PBM. The length of the common channel was 30 mm, and there was a narrow area (arrowhead) in the upper site of confluence of the bile duct and main pancreatic duct. c The histological mapping of the gallbladder cancer. Solid line mucosal carcinoma, open circle invasive cancer, CD cystic duct. d The histological mapping of the bile duct cancer. Solid line mucosal carcinoma, open circle invasive cancer. e H&E specimen of the gallbladder showed papillary carcinoma. CHD common hepatic duct, CD cystic duct, PD an open of the pancreatic duct, Pap papilla of Vater. f The specimen of the bile duct showed invasive well differentiated tubular adenocarcinoma. g, h p53 immunoreactivity was shown in the cancer of gallbladder and the bile duct
a A schematic drawing of the resected specimen, which showed areas of sample collecting. A gallbladder carcinoma occupied the fundic portion of the gallbladder (A). Hyperplastic non-cancerous epithelium was presented at the neck and cystic duct of gallbladder (B). The upper and middle bile duct was extended by obstructive stenosis of lower bile duct lesion. There was no cancerous epithelium (C). Bile duct cancer occupied a limited area of the intra-pancreatic bile duct (D). Mild atypism was present in the common channel away from bile duct cancer (E). No atypical changes showed in the mucosa adjacent to the papilla of Vater (F) and main pancreatic duct (G). b Representative specimen of SSCP and direct sequencing of exon 8 of the p53 gene. Extra peaks on SSCP (arrows) and c non-sense mutation, frame shift of CCA–CA, was present on codon 301 in the gallbladder cancer. d In the bile duct cancer, extra peaks (arrows) and e missense mutation, GTG (Val) to GAG (Glu) was present on codon 272 in bile duct cancer (C)
An extra peak for p53 PCR-SSCP was shown in specimens from both the gallbladder cancer (A) and that of the bile duct (D). The non-sense mutation of CCA (Pro) to CA was found on codon 301 on exon 8 of the p53 gene in the gallbladder cancer (Fig. 2a) and the missense mutation of GTG (Val) to GAG (Glu) was found on codon 272 on exon 8 of the p53 gene in the gallbladder cancer (Fig. 2b). The other sites (B) (C) (E) (F) had no mutation of the p53 gene. No K-ras codon 12 gene mutation was shown at any sites (A)–(F) by PCR-preferential homoduplex formation assay.
To confirm whether the target cells were contained or not in these frozen specimens, the corresponding specimens for each DNA extraction were stained with H&E and p53 immunostaining. They were fixed in acetone and inactivated endogenous peroxidase with 3% hydrogen peroxide and non-specific antibody binding, and then, incubated with DO7 anti-human p53 mouse monoclonal antibody (Dako, Glostrup, Denmark), which recognizes both wild- and mutant-type p53 protein, for 10 min at room temperature. Excess antibody was washed out and incubated with secondary biotinylated anti-rabbit antibodies (LSAB2 Kit, Dako), avidin-biotin complex and 3,3′-diaminobenzidine. Specimens then were counterstained with hematoxylin.
p53 immunostaining was positive on specimens of the gallbladder cancer (A) (Fig. 1g), the lower bile duct cancer (B) (Fig. 1h) and the common channel area (E). Formalin fixed specimens were also used for p53 and Ki-67 (anti-human Ki-67 antibody MIB1, Immunotech, Marseille, France) immunohistochemistry. For p53 labeling, 70% or more of nuclei with an intensity that was clearly demarcated from surrounding mucosa at low power magnification were present in both the gallbladder and bile duct cancer. Moreover, p53 and Ki-67 immunoreactivity was shown on the epithelium of common channel (Fig. 3a–d).
Discussion
The carcinogenetic process in PBM is caused by repeated damage and restoration of biliary epithelium by a mutual countercurrent of pancreatic and bile juice. Regenerated epithelium gradually produces a variant accompanied by cellular atypical change, displaying a hyperplasia–dysplasia–carcinoma sequence [9]. About 30% of patients with dilatation of the bile duct developed carcinogenesis not only in the gallbladder but also the bile duct [10]. Suzuki et al. [11] and Takayashiki et al. [12] reviewed 6 and 12 cases of multiple primary cancer of biliary tract associated with PBM, respectively. They proposed that carcinogenesis of the gallbladder and that of the bile duct were unrelated, on the basis of histological examination. Abnormalities of some oncogenes and cancer suppressor genes occur during each step of carcinogenesis [4–8]. Accumulation of gene abnormality was also important for carcinogenetic process in PBM, because a carcinogenic change was reported from a remnant bile duct or the pancreatic duct in a postoperative case [12]. Gene abnormality of PBM has received little attention. The present case with double primary cancer of the gallbladder and the bile duct is useful to clarify the genetic pathway of carcinogenesis in PBM. In addition, it is extremely valuable to pathologically examine and genetically evaluate abnormalities of the common channel.
The most prevalent K-ras mutation is shown in pancreatic cancer cells (80–100%) and/or metaplastic epithelium of pancreatic duct in cases of chronic pancreatitis [13]. It is suggested that K-ras mutation is an important factor of carcinogenesis related to pancreatic juice [14]. For examination of K-ras in gallbladder mucosa in PBM, K-ras mutation is strongly regarded as an early event in normal to hyperplastic epithelium [14, 15]. Hidaka et al. [14] reported the relation between K-ras mutation of gallbladder mucosa and length of the common channels in case without PBM. Mutation in non-cancerous epithelium was more frequent in long common channels (>5 mm) than in shorter ones. Furthermore, they gradually increased from the upper to lower bile duct. Their results suggested that both the location and the length of the common channel were linked to K-ras mutation and biliary carcinoma. Nagai et al. [16] also showed K-ras mutation in 36.4% of non-cancerous epitheliums of gallbladder, in 58.8% of gallbladder cancers, and in 20% of non-cancerous epithelium in biliary duct and in 62.5% of bile duct cancers. In the present case, the K-ras mutation was not observed at any site of cancer or non-cancerous epithelium. However, for epithelial cells in non-cancerous area with positive Ki-67, it might have caused another signal regulation apart from K-ras gene.
The p53 tumor-suppressor protein is considered to inhibit tumor growth and p53 mutation can be observed in the majority of malignant tumors [7, 8]. p53 induces p21, which causes cell arrest at the G1 period through phosphorylated RB protein, or causes cell death by apoptosis [17]. The most common mechanism of p53 inactivation is missense mutation within exons 5–8 [16]. It is thought that p53 mutation usually occurs in severe dysplasia (carcinoma in situ) or invasive cancer [17]. Matsubara et al. [18, 19] found p53 gene mutation in 75% of the gallbladder cancers and in 35.7 and 16.7% of non-cancerous lesions in the gallbladder and common bile duct, respectively. Kamisawa et al. [20] also showed p53 protein overexpression in non-cancerous epithelium of the gallbladder in patients with a relatively long common channel. Both reports suggest that p53 gene mutations are involved early in the carcinogenesis of biliary epithelium. On the contrary, in the present case p53 gene mutation was recognized only in the gallbladder and bile duct cancer. The role of p53 mutation in the carcinogenesis of PBM requires further clarification.
Nagai et al. [16] reported p53 gene mutation of double cancer of the gallbladder and bile duct and mutation in gallbladder cancer but not in bile duct cancer. They, therefore, concluded that the carcinogenetic processes in the gallbladder and the bile duct differ. In the present study, both the gallbladder cancer and bile duct cancer showed the same pattern of p53 mutation without K-ras mutation. This did not agree with the result of Nagai et al. [16], but suggested that the genetic change may have involved the same pathway in the gallbladder and the bile duct, because the carcinogenic stimuli may be similar.
A relatively long common channel is thought to be an important risk factor for the development of gallbladder cancer [20]. However, reports on carcinogenesis in the common channel epithelium itself are rare, because almost all patients with PBM underwent cholecystectomy or choledochojejunostomy [2]. The structure of the papilla has many anatomic variations in PBM. We focused on the role of the common channel in PBM. Generally, a good correlation has been observed between the missense mutation and accumulation of p53 protein. Non-sense mutation sometimes shows a lack of p53 protein. However, the mutation was present on the exon 8 of p53 gene in the present case. The antibody (DO-7) recognized the epitope which was placed on the upper from the nonsense mutation of p53 gene. It reacts like an epitope in the N-terminus of P53 protein, known to reside between amino acids 35 and 45 [21]. In some cases, a discrepancy is observed when no mutations are detected in cancer cells with p53 protein overexpression [22]. The accumulation of wild-type protein in the present case might reflect a physiological response by p53 to such stimuli as DNA damage [23]. p53 protein accumulated in cells without p53 mutation in the common channel in the present case. If these cells were non-cancerous, they would usually undergo apoptosis; however, the up-regulation of the cell cycle was not related to wild type p53 status. We assumed that other cycle regulatory pathways might be more dominant than the p53 system in the Ki-67 positive epithelium. Cell proliferation is triggered by the binding of growth factors under physiologic conditions. As cell proliferation course except the course of p53, improving reputation of CyclinD [1, 24], TGF-α [25] and COX2 [26] were reported.
Clinically, carcinoma of the papilla of Vater is popular, but, as for the words called the common channel carcinoma, is not familiar. The papilla of Vater is composed of the common channel, the intraduodenal portion of the common bile duct, the intraduodenal portion of the pancreatic duct, and the duodenal mucosa [4]. The clinical cancer tends to occupied astride among them and cannot identify outbreak part closely. Therefore, the cancer of papilla of Vater is diagnosed collectively. Because a common channel in PBM is longer than that in PBM, it is compatible that an initiation of mutagenesis or carcinogenesis occurs in the common channel area widely. Furthermore, the high carcinogenic potential of common channel epithelium in PBM patients is required for part of the carcinogenetic process. According to Younes’ report [27], the p53 accumulation in tumors of the papilla of Vater occurs early in the neoplastic process. Atypical epithelium was found most frequently in the common channel in patients with carcinoma of the papilla of Vater and this would seem to be related to the histological process involved in the development of biliary cancer in the dysplasia–carcinoma sequence [4, 9]. Additional molecular genetic studies are necessary to identify the carcinogenesis in PBM We should pay attention to not only the remnant intra-pancreatic bile duct but the common channel as a potential site for cancer in patients with PBM.
References
Babbitt DP. Congenital choledochal cyst: new etiological concept based on anomalous relationships of common bile duct and pancreatic bulb. Ann Radiol. 1969;12:231–41.
Matsumoto Y, Fujii H, Itakura J, Matsuda M, Nobukawa B, Suda K. Recent advances in pancreaticobiliary maljunction. J Hepatobilialy Pancreat Surg. 2002;9:45–54.
The Japanese Study Group on Pancreaticobiliary Maljunction. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219–21.
Kimura W, Futakawa N, Zhao B. Neoplastic diseases of the papilla of Vater. J Hepatobilialy Pancreat Surg. 2004;11:223–31.
Wistuba II, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobilialy Pancreat Surg. 1999;6:237–44.
Kamel D, Paakko P, Nuorva K, Vahakangas K, Soini Y. p53 and epithelial dysplasias of the gallbladder. J Clin Pathol. 1993;170:67–72.
Wee A, Teh M, Raju GC. Clinical importance of p53 protein in gallbladder carcinoma and its precursor lesions. J Clin Pathol. 1994;47:453–6.
Kimura K, Ohto M, Ono T, Tsuchiya Y, Saisho H, Kawamura K, et al. Congenital cystic dilatation of the common bile duct: relationship to anomalous pancreaticobiliary ductal union. AJR Am J Roentgenol. 1977;128:571–7.
Tsuchida A, Itoi T, Aoki T, Koyanagi Y. Carcinogenic process in gallbladder mucosa with pancreaticobiliary maljunction. Oncol Rep. 2003;10:1693–9.
Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345–51.
Suzuki S, Nakamura S, Ochiai H, Baba S, Sakaguchi T, Tsuchiya Y, et al. Double cancer of the gallbladder and common bile duct associated with an anomalous pancreaticobiliary ductal junction without a choledochal cyst: report of a case. Surg Today. 1999;29:651–5.
Takayashi T, Miyazaki M, Kato A, Ito H, Nakagawa K, Ambiru S, et al. Double cancer of gallbladder and bile duct associated with anomalous junction of the pancreaticobiliary ductal system. Hepatogastroenterology. 2002;49:109–12.
Tsuchida A, Kasuya K, Endo M, Saito H, Inoue K, Nagae I, et al. High risk of bile duct carcinogenesis after primary resection of a congenital biliary dilatation. Oncol Rep. 2003;10:1183–7.
Hidaka E, Yanagisawa A, Seki M, Takano K, Setoguchi T, Kato Y. High frequency of K-ras mutations in biliary duct carcinoma of cases with a long common channel in the papilla of Vater. Cancer Res. 2000;60:522–4.
Tabata T, Fujimori T, Maeda S, Yamamoto M, Saito Y. The role of Ras mutation in pancreatic cancer, precancerous lesions, and chronic pancreatitis. Int J Pancreatol. 1993;14:237–44.
Nagai M, Watanabe M, Iwase T, Yamao K, Isaji S. Clinical and genetic analysis of noncancerous and cancerous biliary epithelium in patients with pancreaticobiliary maljunction. World J Surg. 2002;26:91–8.
Steel RJ, Thompson AM, Hall PA, Lane DP. The p53 tumor suppressor gene. Br J Surg. 1998;85:1460–7.
Matsubara T, Sakurai Y, Zhi LZ, Miura H, Ochiai M, Funabiki T. K-ras and p53 gene mutations in noncancerous biliary lesions of patients with pancreaticobiliary maljunction. J Hepatobilialy Pancreat Surg. 2002;9:312–21.
Matsubara T, Funabiki T, Jinno O, Sakurai Y, Hasegawa S, Imazu H, et al. p53 gene mutations and overexpression of p53 product in cancerous and noncancerous biliary epithelium in patients with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1999;6:286–93.
Kamisawa T, Funata N, Hayashi Y, Egawa N, Nakajima H, Tsuruta K, et al. Pathologic changes in the non-carcinomatous epithelium of the gallbladder in patients with a relatively long common channel. Gastrointest Endosc. 2004;60:56–60.
Leng K, Schlien S, Bosch FX. Refined characterization of head and neck squamous cell carcinoma expressing a seemingly wild-type p53 protein. J Oral Pathol Med. 2006;35:19–24.
Pokroy R, Tendler Y, Pollack A, Zinder O, Weisinger G. p53 expression in the normal murine eye. Invest Ophthalmol Vis Sci. 2002;43:1736–41.
Matsushima AY, Cesarman E, Chadbum A, Knowles DM. Post thymic T cell lymphomas frequently overexpress p53 protein but infrequently exhibit p53 gene mutation. Am J Pathol. 1994;144:573–84.
Itoi T, Shinohara Y, Takeda K, Nakamura K, Takei K, Sanada J, et al. Nuclear cyclin D1 overexpression is a critical event associated with cell proliferation and invasive growth in gallbladder carcinogenesis. J Gastroenterol. 2000;35:142–9.
Kaneko K, Ando H, Ito T, Kasai K, Watanabe Y, Seo T. Increased cell proliferation and transforming growth factor-alpha (TGF alpha) in the gall-bladder epithelium of patients with pancreaticobiliary maljunction. Pathol Int. 1996;46:253–60.
Tsuchida A, Nagakawa Y, Kasuya K, Itoi T, Endo M, Ozawa T, et al. Immunohistochemical analysis of cyclooxygenase-2 and vascular endothelial growth factor in pancreaticobiliary maljunction. Oncol Rep. 2003;10:339–43.
Younes M, Riley S, Genta RM, Mosharaf M, Mody DR. p53 protein accumulation in tumors of the ampulla of Vater. Cancer. 1995;76:1150–4.
Acknowledgments
The authors are indebted to Prof. J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University for his review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kasuya, K., Nagakawa, Y., Matsudo, T. et al. p53 gene mutation and p53 protein overexpression in a patient with simultaneous double cancer of the gallbladder and bile duct associated with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg 16, 376–381 (2009). https://doi.org/10.1007/s00534-008-0030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-008-0030-1