Abstract
Oxygen isotope ratios of well-preserved brachiopod calcite and conodont apatite were used to reconstruct the palaeotemperature history of the Middle and Late Devonian. By assuming an oxygen isotopic composition of −1‰ V-SMOW for Devonian seawater, the oxygen isotope values of Eifelian and early Givetian brachiopods and conodonts give average palaeotemperatures ranging from 22 to 25 °C. Late Givetian and Frasnian palaeotemperatures calculated from δ18O values of conodont apatite are close to 25 °C in the early Frasnian and increase to 32 °C in the latest Frasnian and early Famennian. Oxygen isotope ratios of late Givetian and Frasnian brachiopods are significantly lower than equilibrium values calculated from conodont apatite δ18O values and give unrealistically warm temperatures ranging from 30 to 40 °C. Diagenetic recrystallization of shell calcite, different habitats of conodonts and brachiopods, as well as non-equilibrium fractionation processes during the precipitation of brachiopod calcite cannot explain the 18O depletion of brachiopod calcite. Moreover, the 18O depletion of brachiopod calcite with respect to equilibrium δ18O values calculated from conodont apatite is too large to be explained by a change in seawater pH that might have influenced the oxygen isotopic composition of brachiopod calcite. The realistic palaeotemperatures derived from δ18O apatite may suggest that biogenic apatite records the oxygen isotopic composition and palaeotemperature of Palaeozoic oceans more faithfully than brachiopod calcite, and do not support the hypothesis that the 18O/16O ratio of Devonian seawater was significantly different from that of the modern ocean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The oxygen isotopic composition of biogenic calcite is a powerful proxy to unravel the oxygen isotope ratio of ancient oceans and/or to reconstruct oceanic palaeotemperature and salinity. Brachiopods precipitate shell calcite in near-isotopic equilibrium with ambient seawater (Carpenter and Lohmann 1995; Brand et al. 2003) and have been a preferred tool for isotopic studies because of the diagenetic stability stemming from their primary low-magnesium calcite mineralogy (Bruckschen et al. 1999; Middleton et al. 1991; Mii et al. 1999; Veizer et al. 1986, 1999; Wenzel and Joachimski 1996). Devonian brachiopods were studied by Popp et al. (1986), Brand (1989), Bates and Brand (1991), Gao (1993), Veizer et al. (1986, 1999) and Lee and Wan (2000). Most of these studies concentrated on the investigation of brachiopod shells from specific brachiopod-rich intervals (Popp et al. 1986; Bates and Brand 1991; Gao 1993; Lee and Wan 2000). Veizer et al. (1986, 1999) measured the isotopic composition of Devonian brachiopod shells and compiled an isotope record for the Phanerozoic. The authors observed a secular decrease in the oxygen isotopic composition of biogenic calcite from the present to the Cambrian, and interpreted this trend to reflect a secular change in the 18O/16O ratio of seawater. Brand (1989) focused on the secular evolution of the carbon and oxygen isotopic compositions of Devonian and Lower Carboniferous brachiopod shells and observed significant variations in the δ18O values of Devonian brachiopod calcite with average values ranging from −4.5 to −9.5‰ (V-PDB). Again, the low δ18O values were explained by assuming a lower 18O/16O ratio of Devonian seawater and/or a significant lowering of surface water salinity. However, the hypothesis that the oxygen isotope ratio may have changed during the Phanerozoic and that Devonian seawater was depleted in 18O relative to modern seawater remains controversial (see discussions by Land 1995; Veizer 1995; Muehlenbachs 1998; Lécuyer and Allemand 1999; Wallmann 2001).
Biogenic apatite represents another compound that can be used in order to test the hypothesis of a secular change in the oxygen isotopic composition of seawater. Conodonts are extinct marine early vertebrates (Sanson et al. 1992; Donoghue et al. 2000) that possessed a complex feeding apparatus of elements composed of carbonate-fluor apatite (francolite; Pietzner et al. 1968). The dense microcrystalline structure of conodont apatite is similar to tooth enamel that has been assumed to have a high preservation potential for the primary oxygen isotopic composition. Oxygen isotopic analysis of conodont apatite was previously hampered by the very small size of individual conodont elements, and the large number of elements required for accurate analysis. Consequently, only a few oxygen isotope studies of conodont apatite have been published (Luz et al. 1984; Geitgey and Carr 1987). Microanalytical techniques have enabled us to measure the oxygen isotopic composition of conodont microsamples (≤1 mg). Recently published studies on Silurian and Late Devonian conodonts (Wenzel et al. 2000; Joachimski and Buggisch 2002) showed that conodont apatite can be used to reconstruct high-resolution palaeotemperature curves and that secular changes in the oxygen isotopic composition of seawater inferred from δ18O values of skeletal calcite are not necessarily mirrored in the δ18O values of conodont apatite.
This study focuses on the oxygen isotopic composition of Middle and Late Devonian brachiopod calcite and conodont apatite. The aim of this contribution is to compare the oxygen isotope records of biogenic calcite and apatite with respect to the palaeoclimatic history of this specific time interval and to test the hypothesis of a secular change in the oxygen isotope ratio of seawater.
Samples and analytical methods
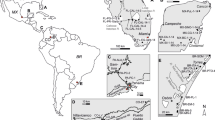
Brachiopods were collected from sections in Morocco (Antiatlas), Spain (Cantabrian Mountains), Germany (Eifel Mountains), North America (Iowa, Manitoba), Siberia (Altai Mountains/Salair area) and South China (Fig. 1). The state of preservation of brachiopod shells was assessed using cathodoluminescence microscopy (CL). Only non-luminescent shells were investigated further by means of trace-element (Sr, Mn, Fe) analysis, and scanning electron microscopy (SEM). Brachiopod shells with well-preserved microstructures, Sr contents higher than 500 ppm, and Fe and Mn contents lower than 400 and 100 ppm, respectively, were classified as diagenetically unaltered (Brand et al. 2003; van Geldern 2004).
Palaeogeographic reconstruction for the Early Devonian (Scotese 2001) and locations of sampled sections for brachiopods (open circles) and conodonts (squares). Samples derive from a latitudinal band of 30°N to 35°S. 1 Iowa Basin (USA); 2 Manitoba (Canada); 3 Rheinisches Schiefergebirge (Germany); 4 Cantabrian Mountains (Spain); 5 Antiatlas (Morocco); 6 Altai-Salair Region (Siberia); 7 China
Stable isotope measurements were performed on calcite powders sampled from well-preserved parts of the shells using a Kiel I carbonate preparation device connected to a ThermoFinnigan 252 mass spectrometer. Oxygen isotope values are reported in ‰ relative to V-PDB by assigning a δ18O value of −2.20‰ to NBS 19. Reproducibility of the oxygen isotope measurements was controlled by replicate analyses of NBS19 and laboratory standards and was better than ±0.06‰ (1σ).
Conodont samples were collected from sections in Germany (Rheinisches Schiefergebirge) and eastern and northern Iowa in central North America (Fig. 1) and processed using standard preparation techniques. Conodont elements (~1 mg) were dissolved in nitric acid and chemically converted to Ag3PO4 using a slightly modified version of the method described by O’Neil et al. (1994). The oxygen isotopic composition was measured on CO generated by reducing trisilverphosphate using a high-temperature conversion-elemental analyzer (TC-EA) connected online to a ThermoFinnigan Delta plus mass spectrometer. Samples and standards were run in triplicate. Accuracy and reproducibility were monitored by multiple analyses of trisilverphosphate prepared from NBS120c and several trisilverphosphate reference samples received from other isotope laboratories. All phosphate δ18O values are reported in ‰ relative to V-SMOW. The overall reproducibility of the apatite oxygen isotope analysis was better than ±0.25‰ (1σ). The mean δ18O value of NBS120c was 22.4‰ V-SMOW which is 0.7‰ higher than the values reported by Crowson et al. (1991) and Lécuyer et al. (1993, 1996), but relatively close to the value of 22.58‰ V-SMOW determined recently by conventional fluorination with BrF5 (Venneman et al. 2002). The oxygen isotopic composition of the Ag3PO4 standard YR-2 (provided by R. Blake and T. Vennemann; see Vennemann et al. 2002) was measured as 13.2‰ V-SMOW. Palaeotemperatures were calculated using the equations given by O’Neil et al. (1969) for carbonate and Kolodny et al. (1983) for apatite. The conversion from the V-PDB to the V-SMOW scale is done by δ18OV-SMOW =1.03091×δ18OV-PDB +30.91 (Coplen et al. 1983).
Results
Oxygen isotopic composition of brachiopod calcite
The oxygen isotopic composition of the well-preserved Middle and Late Devonian brachiopod shells is shown in Fig. 2a. The overall range of the δ18O values is −2.5 to −6.9‰. Eifelian and early Givetian shells record isotope values ranging between −2.5 to −4.0‰, with three values as low as −4.5‰. Average δ18O values were calculated using a 5-point running mean and are around −3.0‰. A decrease in δ18O values starts at the base of the Middle varcus conodont Zone and culminates in the hermanni Zone with values from −6.0 to −6.9‰. During the latest Givetian average δ18O values rapidly increase by more than 2‰ and reveal a second more gradual decrease to values from −5.5 to −6.0‰ during the middle Frasnian (at or near the base of Devonian T-R cycle IIc of Johnson et al. 1985). In the late Frasnian (Late rhenana Zone, at or near the base of the M.N. Zone 13) average δ18O calcite values indicate an increase of almost 1‰ and values around −5.5 to −4.0‰ are registered in the latest Frasnian and early Famennian.
Evolution of Middle to Late Devonian oxygen isotope records measured from brachiopod calcite (A) and conodont apatite (B). Trend lines correspond to 5-point moving average. Conodont biostratigraphy after: late Givetian zones of Klapper and Johnson (1990), Frasnian Montagne Noir Zones 1–13 of Klapper (1989), “Standard” Late Devonian Zones of Ziegler and Sandberg (1990), and Klapper and Becker (1998)
Oxygen isotopic composition of conodont apatite
The oxygen isotope record of Middle and Late Devonian conodonts is shown in Fig. 2b. Eifelian and early Givetian conodonts have δ18O values of 18.5 to 20.5‰ with average values around 19.5‰. A significant shift to lower δ18Oapatite values is observed in the Upper varcus and hermanni zones with values decreasing from 19.3 to 17.6‰. Within the latest Givetian Upper disparilis and norrisi zones the oxygen isotope ratios increase to values around 19.5‰. Subsequently, δ18O apatite values of 19.0 to 19.5‰ are observed in the early Frasnian. The observed δ18O apatite values of the late Frasnian are significantly lower ranging from 17.5 to 19.0‰. The latest Frasnian and the Frasnian-Famennian transition are characterized by two positive δ18Oapatite excursions with amplitudes of 1.0 to 1.5‰. Those two oxygen isotope events coincide with positive excursions in δ13Ccarb and the deposition of the Lower and Upper Kellwasser Horizons, respectively (Joachimski and Buggisch 2002). The δ18O values of early Famennian conodonts cluster around 17.5‰. A minor increase in δ18O to values around 18.0‰ is observed in the middle Famennian.
Discussion
The comparison of the conodont and brachiopod oxygen isotope records documents similar secular trends in the Middle and Late Devonian. We observe relatively high δ18O values in the Eifelian and early Givetian for both conodonts and brachiopods, followed by a pronounced decrease in δ18O values starting in the Middle to Upper varcus Zone that culminates in the hermanni conodont Zone, followed by an increase in δ18O values in the latest Givetian. The oxygen isotopic values of conodont apatite and brachiopod calcite decrease in the Frasnian, but whereas the δ18Oapatite values start to decrease during the upper part of the middle Frasnian (in the hassi Zone, M.N. Zones 7–10), the δ18O record of brachiopod calcite suggests an earlier shift to lower values during the interval of falsiovalis and transitans Zones (M.N. Zones 1–4). Eifelian conodonts and brachiopods are enriched in 18O by approximately 2 to 2.5‰ as compared to Famennian conodonts and brachiopods.
Whereas the general trends in δ18O appear comparable, the magnitudes of the Late Givetian shifts in δ18Oapatite and δ18O calcite differ significantly. The pronounced decrease in δ18O values in the late Givetian is almost –2‰ for conodont apatite, but –3.0 to –3.5‰ for brachiopod calcite. In the latest Givetian, the δ18O values of conodont apatite increase by 2‰ and return to the preceding Eifelian level. The oxygen isotope values of brachiopod calcite also increase by 2‰, but do not reach the early Givetian level.
This discrepancy is better illustrated if the δ18O values are translated into palaeotemperatures, assuming that seawater salinity did not change significantly. Since most of the Devonian is generally accepted as a global greenhouse climatic time period without any evidence for development of a southern hemisphere cryosphere and associated continental ice-sheets, we adopted an oxygen isotopic composition of −1‰ for Devonian seawater (Savin 1977). Palaeotemperatures of 22 to 25 °C are calculated for the Eifelian and early Givetian, from both δ18Oapatite and δ18Ocalcite values (Fig. 3). The decrease in δ18Oapatite in the varcus and hermanni Zones translates into a pronounced palaeotemperature increase with maximum temperatures of 32 °C, but the lowest δ18Ocalcite values give maximum temperatures around 45 °C. Both δ18Oapatite and δ18Ocalcite suggest a significant cooling in the latest Givetian coinciding to the timing of the Givetian-Frasnian bioevent of Walliser (1996) or the Upper disparilis Zone bioevent of Day (1994, 1996). However, the δ18Oapatite values indicate that palaeotemperatures returned to values around 25 °C during the very latest Givetian and early Frasnian whereas palaeotemperatures calculated from δ18Ocalcite are around 33 °C. δ18Oapatite values of the late Frasnian give average temperatures around 29 °C, whereas average temperatures derived from δ18Ocalcite are around 38 °C. Interestingly, the palaeotemperature estimates for the early Famennian are again relatively comparable with temperatures ranging between 30 to 32 °C derived from δ18Oapatite, and temperatures from 32 to 36 °C calculated from δ18Ocalcite. In conclusion, δ18Ocalcite and δ18Oapatite give identical palaeotemperature estimates for the Eifelian and early Givetian and comparable estimates for the early Famennian. However, δ18Ocalcite values from the late Givetian and Frasnian result in unrealistic high palaeotemperatures as compared with the palaeotemperatures calculated from δ18Oapatite.
Palaeotemperature records (5-point moving average) calculated from the oxygen isotope record of conodont apatite (line) and brachiopod clacite (dotted line) in comparison to changes in sea level. Note large differences in the calculated palaeotemperatures in the late Givetian and Frasnian and relative good correspondence in the Eifelian, early Givetian, and the Famennian
Discrepancy between conodont apatite and brachiopod calcite δ18O values
Calcite is expected to be enriched in 18O relative to the oxygen isotopic composition of apatite if both compounds were precipitated from the same solution at a given temperature. The difference in δ18O values depends on temperature and can be calculated using the equations:
For the temperature range of 20 to 40 °C, the calculated offset in δ18O is 8.6 to 9.0‰ and increases by approximately 0.5‰ over the 20 °C temperature range. This theoretically predicted offset is confirmed by measurements of δ18O values of phosphate and structurally bound carbonate in apatite of recent bones and teeth. Bryant et al. (1996) and Iacumin et al. (1996) observed a relatively constant offset in δ18O of 8.6 to 9.0‰, which is expected since the oxygen isotopic composition in mammalian bone phosphate and carbonate is only controlled by variations in the oxygen isotopic composition of the animals body fluid. Consequently, a mean offset of 8.7‰ is taken as the theoretically predicted offset between brachiopod calcite and conodont apatite δ18O values.
The offset in the δ18O values of brachiopod calcite and conodont apatite was calculated by converting the δ18Ocalcite values to the V-SMOW scale and by subtracting the δ18O moving average trend line of conodont apatite from the brachiopod calcite trend line (Fig. 4). As expected, the offset is not constant, but varies by almost 3‰. We observe an average offset of around 8.5‰ for the Eifelian and early Givetian. The difference gradually decreases during the late Givetian by almost 3‰ to around 6.0 to 6.5‰ in the middle Frasnian. In the late Frasnian and early Famennian, the difference between calcite and apatite δ18O values is around 7.7 to 8.0‰. This means that the oxygen isotope values of Eifelian and early Givetian brachiopod calcite and conodont apatite are close to values expected for isotopic equilibrium, whereas the late Givetian and early Frasnian brachiopods have significantly lower δ18O values as compared to those calculated in equilibrium with δ18Oapatite. Alternatively, it could be argued that latest Givetian to Frasnian conodonts are characterized by higher δ18O values than those expected in equilibrium with brachiopod calcite δ18O values.
Difference (Δδ18O) between measured δ18O of brachiopod calcite and conodont apatite. Grey shaded area corresponds to expected Δδ18O in case both compounds were precipitated at identical temperatures and from solutions reflecting the same oxygen isotopic compositions. Note significant deviation from expected Δδ18O in the late Givetian and Frasnian (see text for details)
Diagenesis tends to lower the δ18O values of marine fossils and might be a potential explanation for the observed lower δ18O values of brachiopod calcite. However, a diagenetic overprint of the oxygen isotopic composition of the brachiopod shells was excluded by means of CL, SEM and trace element analysis to the best of our abilities. In addition, it seems unlikely that the varying and smaller than predicted difference between δ18O calcite and δ18O apatite is the consequence of a diagenetic overprint of conodont apatite. Conodont apatite has a dense microcrystalline ultrastructure that is comparable to tooth enamel. It has been shown that tooth enamel is relatively resistant to any diagenetic modification of the primary isotopic composition (Quade et al. 1992; Sharp et al. 2000). Most importantly, diagenesis tends to lower δ18O values as a consequence of increasing burial temperatures and/or post-depositional interactions with meteoric waters depleted in 18O. Consequently, diagenesis is unlikely to account for the observed smaller-than-expected offset in δ18O values of brachiopod calcite and conodont apatite.
Different life habitats of brachiopods and conodonts might be another possible explanation for the observed varying offset in brachiopod and conodont δ18O values. Brachiopods are benthic suspension-feeding organisms and were abundant in subtidal shelfs of Palaeozoic seas. The particulars of the life habitats of the conodont animal have not been firmly established. Whereas some workers suggest that conodonts were living close to the sediment surface, others favour a fully nektonic life style within the water column (Sweet 1988). Since temperature decreases with water depth, the deviation from the expected offset between δ18Ocalcite and δ18O apatite might be interpreted as an effect of different palaeo-water depths (e.g. Picard et al. 1998). However, investigated brachiopod shells record warmer palaeotemperatures in comparison to conodonts. Since benthic brachiopods should record similar or lower temperatures in comparison to the nektobenthic or nektonic conodont animal, the observed discrepancy cannot be explained by a difference in palaeo-water depths.
The comparison of the oxygen isotopic compositions of late Givetian to early Frasnian brachiopods and conodonts from the US Midcontinent (Iowa Basin) supports this interpretation (Fig. 5). The late Givetian to early Frasnian carbonates exposed in Buffalo Quarry (Scott County, Iowa; Day 1994, 1997) represent shallow water deposits that were deposited during Devonian transgressive-regressive cycles IIa-1 to IIb-2 (Day et al. 1996). The oxygen isotopic compositions of brachiopods from the Rapid Member (hermanni to Lower disparilis zones) of the Little Cedar Formation range from −6.7 to −5.3‰ V-PDB (24.0 to 25.4‰ on the V-SMOW scale; see Fig. 5). A significant increase in δ18O values is observed at the top of the Rapid Member with highest δ18Ocalcite values around −4.8‰ (26.0‰ V-SMOW) measured on shells from the Coralville Formation (Upper subterminus Fauna/Upper disparilis Zone). Interestingly, conodonts derived from the same outcrop reflect a comparable trend in δ18O values. Relatively low δ18O apatite values are measured on conodonts from the Rapid Member, and about 1.5 to 1.7‰ higher δ18O apatite values are observed in the early Frasnian. Although conodont apatite and brachiopod calcite record the same trends, the offset between δ18Ocalcite and δ18O apatite is not 8.7‰ as predicted by thermodynamic equilibrium fractionation, but equals approximately 6.5 to 7.0‰ (Fig. 5). The smaller than expected difference between δ18Ocalcite and δ18O apatite can hardly be explained by a deeper water habitat of the conodont animals in comparison to benthic brachiopods since both occupied a subtidal middle shelf epicontinental carbonate ramp on the North American craton. Additionally, we cannot assume that most species in the Devonian Iowa Basin spent most of their lives in deeper water masses of Panthalassa or Palaeotethys oceans and migrated into the vast U.S. Midcontinent sea during the later parts of their life cycles.
Comparison of δ18O values measured on brachiopods and conodonts from Buffalo Quarry (Iowa Basin/USA). δ18O calcite scale was shifted by +8.7‰ in order to account for expected difference between δ18Ocalcite and δ18Oapatite. δ18Ocalcite and δ18Oapatite records reflect identical trends; however, Δδ18O is smaller than predicted by thermodynamic equilibrium fractionation. Unit numbers correspond to units given in Day (1997)
Non-equilibrium fractionation may be another explanation for the observed variations in the oxygen isotope values of brachiopod calcite and conodont apatite. It is generally assumed that brachiopods precipitate calcite of the secondary shell layer in near-isotopic equilibrium with ambient seawater (Carpenter and Lohmann 1995; Brand et al. 2003). However, a high-resolution study of the modern brachiopod Terebratalia transversa by Auclair et al. (2003) revealed a prominent kinetic fractionation effect resulting in a significant depletion in 18O and 13C relative to equilibrium values. Kinetic isotope fractionation results in lower δ18O values of biogenic calcite probably as a consequence of higher reaction rates of isotopically light CO2 during hydration and dehydration reactions prior to carbonate precipitation (McConnaughey 1989). One could argue that certain Devonian brachiopod taxa exhibited a kinetic fractionation effect and that this non-equilibrium fractionation might be responsible for the observed discrepancy.
Lee and Wan (2000) compared the isotopic values of 11 species, 6 genera and 2 families of Middle Devonian brachiopods. The authors observed no species-specific differences in the δ18O and δ13C values and concluded that Devonian brachiopods secreted shell calcite in isotopic equilibrium with ambient seawater. Brachiopods of different families and orders were available for the present study and representatives of each group were investigated from the Middle Devonian as well as from the Frasnian. It is difficult to imagine why the late Givetian and Frasnian representatives should reflect a vital fractionation effect whereas the Eifelian and early Givetian members of the same families precipitated shell calcite in isotopic equilibrium with δ18O values calculated from conodont apatite. Consequently, we argue that non-equilibrium fractionation seems unlikely and does not account for lower δ18O values of late Givetian and Frasnian brachiopod shells.
The fact that we observe comparable trends in the oxygen isotopic composition of conodont apatite and brachiopod calcite suggests that both organisms record the same environmental change. However, the oxygen isotope values of late Givetian and Frasnian brachiopod calcite are lower than equilibrium values calculated from the oxygen isotopic composition of conodont apatite, and translate into unrealistic warm palaeotemperatures. The offset in the δ18O shifts recorded in brachiopod calcite can hardly be explained by non-equilibrium fractionation processes, different life habitats of brachiopods and conodonts, and/or a diagenetic alteration of the primary isotope signals. We speculate that another factor might have been of importance during the precipitation of brachiopod shell calcite. Culture experiments on modern foraminifera (Spero et al. 1997) and inorganic precipitation experiments (McCrea 1950; Usdowski et al. 1991) revealed that seawater carbonate chemistry influences the oxygen isotopic composition of calcite. A decrease in δ18O values of calcite is observed with an increase in pH or increasing CO32- concentrations. Zeebe (1999) proposed that the oxygen isotopic value of total dissolved inorganic carbon (ΣCO2=[CO2]+[H2CO3]+[HCO3−]+[CO32−]) decreases with increasing pH since HCO3−—the predominant carbon species at intermediate pH—is enriched in 18O in comparison to the oxygen isotope ratio of CO32−, the predominant carbon species at high pH. If calcite is formed from a mixture of bicarbonate and carbonate ions in proportion to their relative contribution to total dissolved inorganic carbon, the δ18O value of calcite is expected to decrease with increasing pH (Zeebe 1999). According to Zeebe (1999), an increase of seawater pH of 0.2 to 0.3 units results in a decrease of δ18O values of calcite by 0.22 to 0.33‰. The additional 1 to 1.5‰ negative shift observed in δ18Ocalcite in comparison to δ18Oapatite during the late Givetian could be explained by an increase of seawater pH of approximately 1 to 1.5 units. We are aware of the fact that such an increase in seawater pH seems implausible, and hence this interpretation is speculative given the current state of knowledge. However, if seawater pH increased during the late Givetian, calcite is expected to show a larger decrease in δ18O than coeval apatite.
In summary, we are unable to give a satisfactory explanation for the observed discrepancy in the oxygen isotope records of brachiopod calcite and conodont apatite. The fact that the oxygen isotope values of conodont apatite give more realistic palaeotemperatures for tropical to subtropical surface waters lets us assume that biogenic apatite records Palaeozoic palaeotemperatures more faithfully than brachiopod calcite. Consequently, the conclusions concerning the palaeoclimatic change during the Middle and Late Devonian are based preferentially on the palaeotemperature record derived from the oxygen isotope record of conodont apatite.
Secular change of the oxygen isotope composition of Palaeozoic seawater
The Middle to Late Devonian palaeotemperature record presented in this study for the low latitudes is based on the assumption that the oxygen isotopic composition of seawater during the Devonian, a time period without any significant continental ice sheets, was −1‰ V-SMOW. With this estimate, the oxygen isotope values of Middle and Late Devonian conodont apatite give realistic sea water palaeotemperatures. However, the low δ18O values of late Givetian and Frasnian brachiopods give unrealistically high temperatures. Low δ18O values of Palaeozoic brachiopods that translate into unrealistic high palaeotemperatures initiated the discussion with respect to a potential secular change in the oxygen isotopic composition of seawater from the Cambrian to the present (Veizer et al. 1999). If we assume that Devonian seawater was more depleted in 18O (e.g. δ18Oseawater=−3‰), as suggested by Veizer et al. (1999), the oxygen isotope values of late Givetian and Frasnian brachiopods would give realistic palaeotemperatures. However, the δ18O values of conodont apatite from the late Frasnian and early Famennian, a time interval generally accepted as a very warm climatic period, would result in low palaeotemperatures around 22 °C. In addition, palaeotemperatures calculated from the δ18O values of Eifelian and early Givetian conodonts as well as brachiopods would be around 14 to 16 °C. These temperatures are definitely too low to explain the widespread distribution of Middle Devonian carbonate platforms and reefs in low latitudes. Consequently, we argue that the assumption of a Devonian seawater 18O/16O ratio of −1‰ V-SMOW seems reasonable and that the oxygen isotope values of conodont apatite reflect the environmental conditions more faithfully than the oxygen isotope values of brachiopod calcite. Similar observations were made on Silurian brachiopods and conodonts by Wenzel et al. (2000). Those authors observed comparable trends in the δ18Oapatite and δ18Ocalcite records with the magnitude of the secular δ18O variations measured on Silurian brachiopod calcite being much larger in comparison to the variations recorded by conodont apatite. Low-latitude sea-surface temperatures calculated from δ18O calcite ranged from 24 to 41 °C, whereas δ18Oapatite values gave a minor spread in calculated palaeotemperatures ranging from 26 to 33 °C. The latter were interpreted to represent realistic temperatures for Silurian tropical to subtropical oceanic surface waters. In conclusion, the Silurian δ18Oapatite data presented by Wenzel et al. (2000) and δ18Oapatite values presented in this study seem to be at odds with the hypothesis of a secular change in oxygen isotopic composition of seawater during the Phanerozoic.
Palaeoenvironmental significance of the oxygen isotope record of conodont apatite
The oxygen isotope values of Eifelian to early Givetian conodonts from the Rheinische Schiefergebirge (Germany) give mean palaeotemperatures ranging from 22 to 25 °C. These estimates are similar to those determined by the oxygen isotope values measured on brachiopods from the Eifel (Germany) and Cantabrian Mountains (Spain), as well as from the Antiatlas (Morocco) which translate into mean palaeotemperatures for tropical surface waters of 22 to 23 °C. The δ18Oapatite values of conodonts from Iowa (USA) start to decrease in the Upper varcus Subzone, show lowest values in the hermanni Zone, increase again in the upper part of the hermanni Zone and return to the Eifelian to early Givetian level in the disparilis Zone. This δ18O excursion is currently only documented by conodonts from the US Midcontinent and, as a consequence, we cannot rule out the possibility that this short-term and prominent excursion in δ18O represents a regional signal. The 2‰ lower δ18Oapatite values of conodonts from the hermanni and disparilis Zones in comparison to δ18Oapatite values measured on Eifelian and early Givetian conodonts can be interpreted either as a temperature increase, a salinity decrease or as the combined effect of increasing temperature and decreasing salinity. If the 2‰ decrease is interpreted to result from a change in temperature, late Givetian surface waters of the U.S. Midcontinent (Iowa) would have been up to 8 °C warmer in comparison to Eifelian and early Givetian surface waters of the northern (Rheinisches Schiefergebirge) or southern (Morocco) shelf of the Palaeotethys. Alternatively, enhanced continental runoff and lower salinities of surface waters on the U.S. Midcontinent carbonate shelf may have contributed to the 2‰ decrease in δ18Oapatite. However, a major decrease in surface water salinity would be required if the 2‰ decrease in δ18Oapatite is to be explained exclusively by a lower salinity. This assumption seems implausible since the faunal composition of the carbonates deposited in the Iowa Basin during the latest Givetian do not indicate any major change in salinity (Day et al. 1996). In addition, Palaeozoic brachiopods are considered to have been stenohaline and did not tolerate significant changes in salinity. As a consequence, we preferentially interpret the observed shift in δ18Oapatite as a change in temperature, with a decrease in salinity as a possible subordinate effect. Analysis of coeval intervals from other parts of the world will have to be done in order to demonstrate whether the observed excursion in δ18O is of global significance and whether the decrease in δ18O represents a global warming event.
The lowest δ18Oapatite values that translate into highest palaeotemperatures and/or lower salinities of surface waters in the Iowa Basin are measured on conodonts from the Rapid Member of the Little Cedar Formation which is biostratigraphically contemporaneous with the Geneseo black shale of the Appalachian Basin. The deposition of the Little Cedar Formation was marked by a significant expansion of the seaways on the U.S. Midcontinent carbonate shelf, with open-marine facies spreading across most of Iowa with direct connections with the Cordilleran continental margin through central Canada and an influx of cosmopolitan benthic faunas (Day et al. 1996). The onset of this transgression is dated into the upper part of the Middle varcus Zone, and coincides directly with the Devonian T-R cycle IIa of the sea-level curve of Johnson et al. (1985; = Taghanic onlap of Johnson 1970). However, it is difficult to establish whether the increase in surface water temperature coincided with this prominent transgression since δ18Oapatite data from the Middle-Upper varcus Zones are limited.
Palaeotemperatures calculated for the latest Givetian and early Frasnian are around 25 °C and compare relatively well to temperatures calculated for the Eifelian and early Givetian. Both records (conodont apatite and brachiopod calcite) suggest that the late Frasnian and early to middle Famennian low latitude surface waters were warmer by 8 °C to 10 °C in comparison to the Middle Devonian and early Frasnian surface waters. This warming trend is supported by palaeontological data. According to Streel et al. (2000), tropical (non-equatorial) terrestrial floras extended their range into higher latitudes during the Frasnian and a hot greenhouse climate was inferred for the latest Frasnian when equatorial miospore assemblages seem to have reached their maximum latitudinal distribution. Léthiers and Raymond (1991) conclude that the late Frasnian benthic ostracods may have been adapted to very warm water temperatures. In addition, middle Frasnian metazoan reefs reached 45°N and 30°S of the equator and had a wider latitudinal distribution than Holocene reefs (Copper 2002).
This very warm greenhouse climatic period is interrupted by two short-term cooling events, evidenced by two positive excursions in δ18O of conodont apatite measured in the Late rhenana Zone (at or near the base of M.N. Zone 13) and at the Frasnian-Famennian transition (at or just below the base of the Lower triangularis Zone) that biostratigraphically coincide with the Lower and Upper Kellwasser Horizons (Joachimski and Buggisch 2002). The Kellwasser Horizons represent two short-term anoxic events characterized by positive excursions in carbonate δ13C. Enhanced burial of organic carbon and inferred lowering of atmospheric and oceanic CO2 concentrations were taken as evidence to suggest two short-term episodes of climatic cooling during the latest Frasnian (Joachimski et al. 2002). Indeed, the positive δ18Oapatite excursions indicate cooling of the low latitudes of up to 7 °C. The conodont oxygen isotope data presented in this paper—in conjunction with the data presented by Joachimski and Buggisch (2002)—suggest that during the Late Frasnian and early to middle Famennian tropical to subtropical surface waters may have been much warmer in comparison to the Middle Devonian, and warmer than modern tropical to subtropical surface waters. These warm surface water conditions in conjunction with two superimposed short-term cooling events may have had a severe impact on the low-latitude shallow-water faunas that were severely decimated during the Frasnian-Famennian mass extinction event.
Conclusions
The δ18O values of brachiopod calcite and conodont apatite were measured in order to reconstruct the palaeotemperature history for the Middle and Late Devonian time interval. Eifelian and early Givetian low-latitude sea-surface temperatures derived from both oxygen isotope records are around 23 to 25 °C. A major warming event is indicated by the conodont δ18O record to occur in the middle Frasnian with palaeotemperatures reaching 30 to 32 °C in the late Frasnian. Average Frasnian palaeotemperatures calculated from δ18O values of brachiopod calcite range from around 35 to 40 °C and are close to or above the lethal thermal limit for marine invertebrates (Brock 1985). The discrepancy in the δ18O values of late Givetian and Frasnian brachiopod calcite and conodont apatite can currently not be explained satisfactorily. Neither diagenetic alteration, nor non-equilibrium isotope fractionation, nor different life habitats of brachiopods and conodonts can account for the observed minor offset between δ18O calcite and δ18Oapatite in comparison to the thermodynamically predicted difference. The fact that the δ18O values of conodont apatite translate into realistic palaeotemperatures lets us assume that conodont apatite records Palaeozoic temperatures more faithfully than brachiopod calcite.
References
Auclair AC, Joachimski MM, Lécuyer C (2003) Deciphering kinetic, metabolic and environmental controls on stable isotope fractionations between seawater and the shell of Terebratalia transversa (Brachiopoda). Chem Geol 202:59–78
Bates NR, Brand U (1991) Environmental and physiological influences on isotopic and elemental compositions of brachiopod shell calcite: implications for the isotopic evolution of Palaeozoic oceans. Chem Geol 94:67–78
Brand U (1989) Global climatic changes during the Devonian-Mississippian: stable isotope biogeochemistry of brachiopods. Palaeogeogr Palaeoclimatol Palaeoecol 75:311–329
Brand U, Logan A, Hiller N, Richardson J (2003) Geochemistry of modern brachiopods: applications and implications for oceanography and palaeoceanography. Chem Geol 198:305–334
Brock TD (1985) Life at high temperatures. Science 230:132–138
Bruckschen P, Oesmann S, Veizer J (1999) Isotope stratigraphy of the European Carboniferous: proxy signal for ocean chemistry, climate and tectonics. Chem Geol 161:243–264
Bryant JD, Koch PL, Froelich PN, Showers WJ, Genna BJ (1996) Oxygen isotope partitioning between phosphate and carbonate in mammalian apatite. Geochim Cosmochim Acta 60:5145–5148
Carpenter SJ, Lohmann KC (1995) δ18O and δ13C values of modern brachiopod shells. Geochim Cosmochim Acta 59:3749–3764
Coplen TB, Kendall C, Hopple J (1983) Comparison of isotope reference samples. Nature 302:236
Copper P (2002) Reef development at the Frasnian/Famennian mass extinction boundary. Palaeogeogr Palaeoclimatol Palaeoecol 181:27–65
Crowson RA, Showers WJ, Wright EK, Hoering TC (1991) Preparation of phosphate samples for oxygen isotope analysis. Anal Chem 63:2397–2400
Day J (1994) Late Middle and Early Upper Devonian brachiopod faunas of southeastern Iowa and northwestern Illinois. In: Bunker BJ (ed) Palaeozoic stratigraphy of the Quad-Cities Region East-Central Iowa, Northwestern Illinois. Geol Soc Iowa Guidebook 59:65–83
Day J (1996) Faunal signatures of Middle-Upper Devonian depositional sequences and sea level fluctuations in the Iowa Basin: US mid-continent. Geol Soc Am Spec Pap 306:277–300
Day J (1997) Phylogeny and biogeography of Tecnocyrtina (Brachiopoda-Spiriferinida) in the Devonian (Givetian-Frasnian) of North America. Geol Soc Am Spec Pap 321:245–261
Day J, Uyeno T, Norris W, Witzke BJ, Bunker BJ (1996) Middle-Upper Devonian relative sea-level histories of central and western North American interior basins. Geol Soc Am Spec Pap 306:259–275
Donoghue PCJ, Forey PL, Aldridge RJ (2000) Conodont affinity and chordate phylogeny. Biol Rev 75:191–251
Gao G (1993) The temperature and oxygen-isotopic composition of early Devonian oceans. Nature 361:712–714
Geitgey JE, Carr TR (1987) Temperature as a factor affecting conodont diversity and distribution. In: Austin RL (ed) Conodonts: investigative techniques and applications. Elishorwood, Chichester, pp 242–255
Iacumin P, Bocherens H, Mariotti A, Longinelli A (1996) Oxygen isotope analysis of co-existing carbonate and phosphate in biogenic apatite: a way to monitor diagenetic alteration of bone phosphate? Earth Planet Sci Lett 142:1–6
Joachimski MM, Buggisch W (2002) Conodont apatite δ18O signatures indicate climatic cooling as a trigger of the Late Devonian (F-F) mass extinction. Geology 30:711–714
Joachimski MM, Pancost RD, Freeman KH, Ostertag-Henning C, Buggisch W (2002) Carbon isotope geochemistry of the Frasnian-Famennian transition. Palaeogeogr Palaeoclimatol Palaeoecol 181:91–109
Johnson JG (1970) Taghanic onlap and the end of North American provinciality. Geol Soc Am Bull 81:2077–2106
Johnson JG, Klapper G, Sandberg CA (1985) Devonian eustatic fluctuations in Euramerica. Geol Soc Am Bull 96:567–587
Klapper G (1989) The Montagne Noire Frasnian (Upper Devonian) conodont succession. In: McMillan NJ, Embry AF, Glass DJ (eds) Devonian of the world. Can Soc Petrol Geol Mem 14:3:449–468
Klapper G, Becker RT (1998) Comparison of Frasnian (Upper Devonian) conodont zonations. Bull Della Soc Palaeontol Ital 37(2–3):339–348
Klapper G, Johnson JG (1990) Revision of Middle Devonian conodont zones. J Palaeontol 64:902–941
Kolodny Y, Luz B, Navon O (1983) Oxygen isotope variations in phosphate of biogenic apatites, I. Fish bone apatite—rechecking the rules of the game. Earth Planet Sci Lett 64:398–404
Land LS (1995) Oxygen and carbon isotopic composition of Ordovician brachiopods: implication for coeval sea water: discussion. Geochim Cosmochim Acta 40:2843–2844
Lécuyer C, Allemand PA (1999) Modelling of the oxygen isotope evolution of seawater: implications for the climate interpretation of the δ18O of marine sediments. Geochim Cosmochim Acta 63:351–361
Lécuyer C, Grandjean P, Emig CC (1996) Determination of oxygen isotope fractionation between water and phosphate from living linguilides: potential application to palaeoenvironmental studies. Palaeogeogr Palaeoclimatol Palaeoecol 126:101–108
Lécuyer C, Grandjean P, O’Neil JR, Capetta H, Martineau F (1993) Thermal excursions in the ocean at the Cretaceous-Tertiary boundary (northern Morocco): δ18O record of phosphatic fish debris. Palaeogeogr Palaeoclimatol Palaeoecol 105:235–243
Lee X, Wan G (2000) No vital effect on δ18O and δ13C values of fossil brachiopod shells, Middle Devonian of China. Geochim Cosmochim Acta 64:2649–2664
Léthiers F, Raymond D (1991) Les crises du Dévonien supérieur par l’étude des faunes d’ostracodes dans leur cadres paléogeographiques. Palaeogeogr Palaeoclimatol Palaeoecol 88:133–146
Luz B, Kolodny Y, Kovach J (1984) Oxygen isotope variations in phosphate of biogenic apatites, III. Conodonts. Earth Planet Sci Lett 69:255–262
McConnaughey T (1989) 13C and 18O disequilibrium in biological carbonates: I. Patterns. Geochim Cosmochim Acta 53:151–162
McCrea JM (1950) On the isotopic geochemistry of carbonates and a palaeotemperature scale. J Chem Phys 18:849–857
Middleton PD, Marshall JD, Brenchley PJ (1991) Evidence for isotopic changes associated with the Late Ordovician glaciation from brachiopods and marine cements from central Sweden. In: Barnes CR, Williams SH (eds) Advances in Ordovician geology. Geol Surv Can Pap 90–9, Ottawa, pp 313–323
Mii HS, Grossman EL, Yancey TE (1999) Carboniferous isotope stratigraphies of North America: implications for Carboniferous palaeoceanography and Mississippian glaciation. Geol Soc Am Bull 111:960–973
Muehlenbachs K (1998) The oxygen isotopic composition of the oceans, sediments and the sea floor. Chem Geol 145:263–273
O’Neil JR, Clayton RN, Meyeda TK (1969) Oxygen isotope fractionation in divalent metal carbonate. J Chem Phys 51:5547–5558
O’Neil JR, Roe JL, Reinhardt E, Blake RE (1994) A rapid and precise method of oxygen isotope analysis of biogenic phosphate. Isr J Earth Sci 43:203–212
Picard S, Garcia JP, Lécuyer C, Sheppard SMF, Cappeta H, Emig CC (1998) δ18O values of coexisting brachiopods and fish: temperature differences and estimates of palaeo-water depths. Geology 26:975–978
Pietzner H, Vahl J, Werner H, Ziegler W (1968) Zur chemischen Zusammensetzung und Mikromorphologie der Conodonten. Palaeontographica Abt A 128:115–152
Popp BN, Anderson TF, Sandberg PA (1986) Brachiopods as indicators of original isotopic compositions in some Palaeozoic limestones. Geol Soc Am Bull 97:1262–1269
Quade J, Cerling TE, Barry JC, Morgan ME, Pilbeam DR, Chivas AR, Lee-Thorp JA, van der Merve NJ, (1992) A 16-Ma record of palaeodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. Chem Geol 94:183–192
Sanson IJ, Smith MP, Amstrong HA, Smith MM (1992) Presence of the earliest vertebrate hard tissues in conodonts. Science 256:1308–1311
Savin SM (1977) The history of the earth’s surface temperature during the past 100 million years. Ann Rev Earth Planet Sci 5:319–355
Scotese CR (2001) Atlas of earth history, vol 1, palaeogeography. PALEOMAP Project, Arlington, Texas, 52 pp
Sharp ZD, Atudorei V, Furrer H (2000) The effect of diagenesis on oxygen isotope ratios of biogenic phosphates. Am J Sci 300:222–237
Spero HJ, Bijma J, Lea DW, Bemis BE (1997) Effect of seawater carbonate concentration on Foraminiferal carbon and oxygen isotopes. Nature 390:497–500
Streel M, Caputo MV, Loboziak S, Melo JHG (2000) Late Frasnian-Famennian climates based on palynomorph analyses and the question of the Late Devonian glaciations. Earth Sci Rev 52:121–173
Sweet WC (1988) The conodonta: morphology, taxonomy, palaeoecology and evolutionary history of a long-extinct animal phyllum. Oxford Mon Geol Geophys 10:1–212
Usdowski E, Michaelis J, Böttcher M, Hoefs J (1991) Factors for the oxygen isotope equilibrium fractionation between aqueous and gaseous CO2, carbonic acid, bicarbonate, carbonate, and water (19 °C). Z Phys Chem 170:237–249
Van Geldern R (2004) Stabile Isotopenuntersuchungen an devonischen Brachiopoden. Unpublished PhD Thesis, University of Erlangen-Nürnberg, pp 210
Veizer J (1995) Oxygen and carbon isotopic composition of Ordovician brachiopods: implication for coeval sea water: reply. Geochim Cosmochim Acta 59:2845–2846
Veizer J, Ala D, Azmy K, Bruckschen P, Buhl D, Bruhn F, Carden GAF, Diener A, Ebneth S, Godderis Y, Jasper T, Korte C, Pawellek F, Podlaha OG, Strauss H (1999) 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem Geol 161:59–88
Veizer J, Fritz P, Jones B (1986) Geochemistry of brachiopods: oxygen and carbon isotopic records of Palaeozoic oceans. Geochim Cosmochim Acta 50:1679–1696
Vennemann TW, Fricke HC, Blake RE, O’Neil JR, Colman A (2002) Oxygen isotope analysis of phosphates: a comparison of techniques for analysis of Ag3PO4. Chem Geol 185:321–336
Walliser OH (1996) Global events in the Devonian and Carboniferous. In: Walliser OH (ed) Global events and event stratigraphy. pp 225–250
Wallmann K (2001) The geological water cycle and the evolution of marine δ18O values. Geochim Cosmochim Acta 65:2469–2485
Wenzel B, Joachimski MM (1996) Carbon and oxygen isotopic composition of Silurian brachiopods (Gotland/Sweden): palaeoceanographic implications. Palaeogeogr Palaeoclimatol Palaeoecol 122:143–166
Wenzel B, Lécuyer C, Joachimski MM (2000) Comparing oxygen isotope records of Silurian calcite and phosphate - δ18O compositions of brachiopods and conodonts. Geochim Cosmochim Acta 64:1859–1872
Zeebe RE (1999) An explanation of the effect of seawater carbonate concentration on Foraminiferal oxygen isotopes. Geochim Cosmochim Acta 63:2001–2007
Ziegler W, Sandberg CA (1990) The Late Devonian standard conodont zonation. Courier Forschungsinstitut Seckenberg 121:1–115
Acknowledgements
We thank B. Wenzel and D. Lutz for help with conodont apatite δ18O analyses. This study was financially supported by the Deutsche Forschungsgemeinschaft (Projects Bu 312/31 and Jo 312/4). Brachiopods were provided by F. Alvarez, E.A. Yolkin, X. Ma, and U. Jansen. Funding for parts of Day’s travel for field work with M. M. Joachimski and R. van Geldern in Iowa in 1999 was provided by an Illinois State University – University research grant, and by the Foundation for Research and Exploration of the National Geographic Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joachimski, M.M., van Geldern, R., Breisig, S. et al. Oxygen isotope evolution of biogenic calcite and apatite during the Middle and Late Devonian. Int J Earth Sci (Geol Rundsch) 93, 542–553 (2004). https://doi.org/10.1007/s00531-004-0405-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00531-004-0405-8