Abstract
Purpose
We assessed the efficacy of aprepitant (APR) or 10 or 5 mg OLN (OLN10 or OLN5) plus ondansetron and dexamethasone for chemotherapy-induced nausea/vomiting (CINV) prophylaxis in patients receiving high-emetogenic chemotherapy (HEC).
Methods
Patients who received doxorubicin + cyclophosphamide or cisplatin were given intravenous ondansetron and dexamethasone prior to chemotherapy and oral dexamethasone on days 2–4 and randomized 1:1:1 to receive APR125 on day 1 and APR80 on days 2–3 or OLN10 or OLN5 on days 1–4. Matched placebo controls were used. The primary endpoint was no nausea in ≤ 120 h. Secondary endpoints included CINV severity, complete response (CR) rate, adverse effects (AE), and quality of life.
Results
Of 141 patients, 104 received AC and 37 received cisplatin. The no-nausea rates were 33% (APR), 43.2% (OLN10; p = 0.24), and 37% (OLN5; p = 0.87). Grades 2–4 nausea were experienced by fewer patients for OLN10 than for APR (24–120 h, 8.7% vs. 27.7%, respectively; p = 0.02; overall period, 19.6% vs. 40.4%, respectively; p = 0.03). The median visual analog scale nausea score from 24 to 120 h was significantly lower for OLN10 (2.3) than for APR (1.2, p = 0.03). The degrees of vomiting, CR, and AE were similar between the APR and OLN10 groups. CINV was similar between the OLN5 and APR groups.
Conclusions
Nausea was less severe for OLN10 than for APR in patients receiving HEC, but other measures were similar. CINV prevention efficacy was comparable between OLN5 and APR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the most feared adverse effects (AE). As a result, patients occasionally deny accepting crucial chemotherapy for fear of nausea/vomiting. Some patients experience severe vomiting or prolonged nausea, which may be a cause of anticipatory emesis in subsequent cycles. High-emetogenic chemotherapy (HEC), including cisplatin at a dose ≥ 50 mg/m2 or doxorubicin plus cyclophosphamide (AC), causes CINV in > 90% of patients without proper emetic prophylaxis. Ondansetron is the only reimbursable 5-hydroxytryptamine 3 (5-HT3) antagonist in Thailand and is relatively available. There is limitation in usage of other 5-HT3 antagonist, i.e., palonosetron, in Thailand because of high cost and reimbursement issue. Only 25–36% of patients receiving high-dose cisplatin with ondansetron and dexamethasone prophylaxis have achieved complete response (CR), defined as no episode of vomiting and no rescue treatment required within 120 h [1,2,3]. Additionally, only 10–25% of patients have reported no nausea over any time period [1, 4].
Presently, there has been remarkable improvement in CINV prophylaxis. Two major regimens of antiemetics for HEC, including neurokinase 1 (NK1) receptor antagonist or olanzapine (OLN)–based regimen, have been proved of their superiority to an old-fashion regimen of a serotonin uptake inhibitor (5-HT3 antagonist) combined with a steroid [5,6,7,8,9]. Most of these studies assessed the benefit of adding an NK1 receptor antagonist or OLN to long-acting palonosetron. Several studies have demonstrated the efficacy of OLN for CINV prophylaxis when combined with a 5-HT3 antagonist plus a steroid or an NK1 receptor antagonist–based regimen [5, 10, 11]. Recently, investigators from two academic centers in Thailand confirmed the efficacy of OLN added to ondansetron and showed better CINV control in the acute, delayed, and overall periods than that for ondansetron and dexamethasone [3, 4]. Addition of OLN has increased CR rate from 25 to 68% among breast cancer patients receiving the AC regimen [4]. Regarding the interaction between ondansetron and OLN, no case with clinically significant cardiac complications of QT prolongation or arrhythmia has been reported [3].
A previous trial in 2011 directly compared aprepitant (APR) with OLN when used with palonosetron and dexamethasone [12], and OLN provided better nausea control. This finding was also confirmed in a network meta-analysis in 2018 [13]. However, information on the efficacy of APR versus OLN is scarce, especially when these drugs are combined with ondansetron, which is the only re-imbursable 5-HT3 antagonist in Thailand. Thus, a clinical trial of adding either APR or OLN to ondansetron in a Thai population or in patients in limited-resource countries would be beneficial. Although OLN may theoretically interact with ondansetron causing QT prolongation, OLN combined with ondansetron has recently been approved to prevent CINV associated with HEC in Thailand. The recommended dosage of OLN is 10 mg orally prior to chemotherapy administration and then 10 mg on days 2–4. Given the relatively smaller body build of Thai patients relative to that of Western patients, we wanted to compare the frequently used dosage of 5 mg OLN (OLN5) with an APR-based regimen.
This study aims to compare the efficacies of APR and two dosages of OLN when combined with ondansetron and dexamethasone for emetic prevention in patients receiving high-dose cisplatin or AC.
Patients and methods
Study design
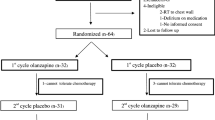
This randomized, double-blind, placebo-controlled study assessed the efficacy and safety of OLN 10 mg (OLN10) or OLN 5 mg (OLN5) or APR when each was combined with ondansetron and dexamethasone for CINV prevention in patients receiving HEC at the Siriraj Hospital between February 2019 and December 2019. Eligible patients were randomized to one of three treatment arms at a 1:1:1 ratio by computer-generated mixed blocks of 2, 4, 6, and 8 allocation schedules. Randomization was stratified by chemotherapy regimen, e.g., cisplatin > 50 mg/m2 or doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2 (AC). All patients were followed up for 5 days. APR and placebo were encapsulated in indistinguishable capsules by a pharmacist who was blinded to the randomization sequence. OLN and indistinguishable placebo were prepared by a blinded pharmacist. The trial protocol was approved by the Siriraj Institutional Review Board and supported by a grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Grant Number (IO) R016231016. All recruited patients provided written inform consent.
Patients
Eligible patients were chemo-naïve, ≥ 18 years old with a confirmed solid malignancy, and scheduled to receive a first dose of cisplatin > 50 mg/m2 or AC regimen. Cisplatin can be given concurrently with radiotherapy or combined with other chemotherapy. Other moderately or highly emetogenic chemotherapy was allowed if it was to be used only on the same day of cisplatin. All patients were required to have creatinine clearance > 50 ml/min and < 2 times the upper normal limit of aminotransferase. Exclusion criteria included prior chemotherapy use, pregnancy, an episode of vomiting within 24 h, untreated gut obstruction, uncontrolled brain metastasis, significant heart disease, or previous use of OLN or APR. Additionally, patients with a known allergy to ondansetron or who had contraindications to steroids were excluded from the study.
Treatments
All patients, regardless of their study arm, received intravenous 8 mg of ondansetron and 12 mg of dexamethasone 30 min before chemotherapy administration, followed by dexamethasone 8 mg orally on days 2–4. The addition of OLN10 or OLN5 or APR was performed in a blinded double-dummy fashion, as detailed in Table 1. Rescue treatment was permitted at the primary physician’s discretion. Breaking the code was allowed if there was a request from a primary physician so that they could treat existing symptoms or plan for prophylactic regimen in the next cycle.
Assessment
During the 0–120 h after chemotherapy infusion, all patients were required to complete a daily record of episodes of vomiting, degree of nausea, and rescue treatment. Additionally, adverse events and quality of life (QOL), assessed by the Functional Living Index Emesis (FLIE), were scheduled on day 5.
On day 2 after chemotherapy administration, assessors who were blinded to the study arms visited the patients who received cisplatin and remained hospitalized or made a telephone call to others who were treated as outpatients to collect data as follows: emetic episode, frequency of emesis, degree of nausea, adverse events, and rescue treatment. Moreover, the assessors ensured compliance of the patients with the allocated medication and reminded them to complete the questionnaire. On day 5, the patients were telephoned to collect the aforementioned parameters of days 2–5 and remind them to return the questionnaire by mail.
The questionnaire comprised two parts: self-reported degree of nausea and vomiting, including rescue treatment and QOL. In addition to the assessors’ interview, the patients were required to complete their report regarding nausea and vomiting grade according to the Common Toxicity Criteria and visual analog scale (VAS) nausea score. The FLIE questionnaire is a validated nausea- and vomiting-specific patient-reported QOL measurement that consists of nine items in the nausea domain and nine items in the vomiting domain. Responses to each item were marked with a vertical mark on the VAS ranging from 0 to 7 representing “none/not at all” and “a great deal” by the patients on day 5. The interpretation of FLIE was performed according to the licensor’s recommendation [14].
The severities of nausea and vomiting were classified according to the Common Terminology Criteria for Adverse Events version 3.0. We focused more on the degree of nausea assessed by the VAS nausea score ranging from 0 to 10. No nausea was assigned a score of 0, and the most severe nausea was assigned a score of 10. CINV was reported as acute (0–24 h after chemotherapy), delayed (24–120 h after chemotherapy) phase, and overall period (0–120 h). Severities of nausea and vomiting were reported as the worst grade from either the assessor’s interview or the self-reported grade and the worst VAS scores during the 0–24 h, 24–120 h, and overall periods.
Statistical analysis
The primary hypothesis was that OLN10 would be superior to APR in terms of the no-nausea rate. Based on the hypothesis of a no-nausea rate improvement from 38 to 70% among patients who received APR and OLN [12], 43 evaluable patients per treatment group were required to achieve 80% power and two-sided alpha error of 0.05. Since OLN5 is commonly used in clinical practice based on its lower toxicity profile, especially sleepiness, OLN5 was assigned as another treatment arm to compare it with APR. To account for a dropout rate of 10%, the accrual targets were adjusted to a total of 47 patients in each arm.
The primary endpoint was a no-nausea rate, defined by a VAS nausea score of 0. The secondary endpoints included degree of nausea or vomiting, VAS nausea score, CR rate, rescue treatment, AE, and QOL. CR was defined as no episode of emesis and no rescue treatment required during a 5-day period. The regimens of OLN10 or OLN5 were compared with APR, which was considered to be the control group.
All demographic data were reported by using descriptive statistics. The no-nausea rate, degree of nausea/vomiting, proportion of patients with CR or requiring rescue treatment, and AE were analyzed by performing Pearson’s chi-square test. The VAS nausea scores were compared by performing the independent t test. All analyses were based on an intention to treat basis. Two-sided p values are used throughout this report. All statistical analyses were performed by using SPSS version 18 (SPSS Statistics, IBM Corp. Armonk, NY, USA).
Results
Patient characteristics
From February to December 2019, 141 patients were enrolled in the study. There were 47, 46, and 47 patients assigned to receive APR, OLN10, and OLN5, respectively. The baseline characteristics of the patients in the treatment groups were similar (Table 2). The majority of the patients was female and treated with a first cycle of AC for breast cancer therapy. Of 37 patients receiving high-dose cisplatin, most of the patients received single-agent cisplatin concurrently with radiotherapy. Other combined chemotherapy administration included 5-fluorouracil, gemcitabine, and vinorelbine. The mean doses of cisplatin were comparable among all treatment groups (91.5, 90.9, and 90.5 mg/m2 for the patients given APR, OLN10, and OLN5, respectively). Body surface areas were identical among all treatment groups.
Antiemetic efficacy
Efficacy of nausea control is shown in Table 3. A non-significantly higher proportion of patients given OLN10 (43.2%) or OLN5 (37%) experienced no-nausea during a 5-day period relative to those given APR (33.3%). Interestingly, a significantly lower proportion of patients in the OLN10 group had moderate to severe nausea, defined as grades 2–4 nausea according to the Common Terminology of Adverse Events, relative to the proportion in the APR group (8.7% vs. 27.7%, respectively; p = 0.02). Meaningful better nausea control of OLN10 was demonstrated regardless of chemotherapy types. Patients in the OLN5 group had a degree of nausea rate comparable with that of the APR group. The degree of nausea assessed by the VAS was significantly lower in the OLN10 group than in the APR group during the delay (1.2 vs. 2.3, respectively; p = 0.03) and overall periods (1.3 vs. 2.3, respectively; p = 0.05). However, the VAS nausea scores were similar between the OLN5 and APR groups (Fig. 1).
The degrees of vomiting were not different among the treatment arms in the acute, delayed, and overall periods (Table 4). Additionally, the CR rates of the patients in the OLN10 (65.2%, p 0.94) and OLN5 (68%, p 0.83) groups were not significantly different than that in the APR group (66%). Six (12.8%) patients in the APR group and five (10.9%) and four (8.5%) patients in the OLN10 and OLN5 groups, respectively, required rescue treatment.
Adverse events
The most common AE were anorexia and fatigue, followed by constipation, hiccups, and bloating. OLN10 was generally well tolerated. Patients receiving OLN10 experienced an insignificantly greater proportion of sleepiness than that of the APR group. No severe adverse events were reported. Moreover, the lower dose of OLN5, which is the most commonly used regimen in Thailand, was not associated with a lesser proportion of patients who reported sleepiness. Overall, no significant safety concerns were identified among the three treatment arms (Table 5).
QOL
We assessed QOL by administering a FLIE questionnaire and asked the patients to return the questionnaires by mail. Unfortunately, we obtained evaluable responses from only 99 (70%) patients. Table 6 shows similar proportions of patients who reported no effects of nausea, vomiting, and CINV on quality of daily life between the treatment arms.
Discussion
Ondansetron is a first-generation 5-HT3 antagonist that is the only re-imbursable drug in this class in Thailand and is widely accessible. Compared with palonosetron, ondansetron has a shorter half-life and greater chance of drug interaction with OLN, possibly causing QT prolongation. Recent randomized controlled trials have demonstrated a benefit from addition of OLN to conventional ondansetron and dexamethasone, as shown by an increased CR rate and improved safety [3, 4]. The present study is the first randomized, double-blind, placebo-controlled study that has evaluated the standard dose of OLN10 versus APR with addition of ondansetron plus dexamethasone for HEC. Several prospective trials have compared OLN with NK1 receptor antagonists and showed the superiority of OLN in terms of nausea control [12, 13, 15]. However, this comparison has not been made between the most commonly used regimen consisting of ondansetron and dexamethasone in Thailand.
Similar to previous studies, we demonstrated improvement in the degree of nausea control between patients receiving OLN10 and APR. Navari et al. reported no-nausea rates of 69% and 38% among patients who received OLN10 and APR, respectively [12]. In our study, which recruited a similar patient population, only 43% of the patients in the OLN10 group and 33% of the patients in the APR group experienced no-nausea during a 5-day period. Two-thirds of the patients in our study achieved CRs, whereas almost all patients in Navari’s study did, which shows that our population had worse nausea control than that study. However, the no-nausea and CR rates in our study were comparable with those in studies from other institutions in Thailand that used ondansetron as the backbone antiemetic agent [3, 4]. The differences in antiemetic efficacy and magnitude of benefit of OLN between our study and the Navari’s study are possibly explained by the different backbone of the 5-HT3 antagonist used in the studies in Thailand.
Although our study failed to show a significant benefit of OLN10 in the no-nausea rate as a primary endpoint, there was a trend toward a 10% improvement in the no-nausea rate (33% vs. 43%) and the study showed a significant benefit in terms of a lower degree of nausea in patients treated with OLN10. A possible reason for our nausea result is that OLN may not add as much benefit to ondansetron, which has a relatively lower antiemetic potency than that of palonosetron, which was used in the previous study. OLN10 may have provided some improvement in the no-nausea rate but not enough to reach statistical significance. Another possible reason for our result is an inadequate sample size, which was calculated based on assuming a substantial benefit in nausea control based on a previous study using palonosetron which has a longer half-life and greater antiemetic potency than for ondansetron. Our trial had sufficient power to show substantial benefits. A larger study would be required to demonstrate a moderate benefit of OLN10, which might still be clinically meaningful.
Regarding AE, OLN10 was associated with a higher number of patients who experienced sleepiness, but the difference was not statistically significant, and the effect did not lead to impairment of QOL. Considering economic issues, OLN costs about one-tenth of the cost of APR (279 THB vs. 2917 THB per cycle). A previous cost analysis of OLN compared with doublet antiemetics in Southeast Asian countries supported the addition of OLN to the standard doublet regimen of a 5HT3 antagonist and a steroid [16]. Taken together, OLN10 is efficacious, well-tolerated, and less costly when compared with an APR-based antiemetic regimen.
Another important comparison in this study was OLN5 (5 mg) versus APR. A previous study from Japan showed antiemetic potency of OLN5 similar to that of OLN10, with more favorable AE, when used in addition to palonosetron and an NK1 receptor antagonist [17]. Again, data regarding OLN5 with ondansetron or compared with an NK1 receptor antagonist is scarce. We showed no significant differences in CINV control or adverse events between patients receiving OLN5 and APR. Although this study did not compare two dosages of OLN, the results suggest that OLN10 might provide better nausea control than that of OLN5 when used with ondansetron. The lower dose of OLN5 is more commonly used in Thailand because most physicians are concerned about the somnolent effect of OLN10. In our study, about 40% of the patients in the OLN5 and OLN10 groups reported sleepiness after treatment, and similar percentages of patients without impact to QOL. There were no differences in the percentages of other adverse events between the treatment groups.
The strength of this study is that it provides a holistic assessment of antiemetic efficacy, AE, and QOL of aprepitant and two dosages of olanzapine that should provide practical results useful in clinical practice. The main limitation of the study is that some data were missing from the VAS nausea scores, which were used as our primary endpoint, and from some of the QOL questionnaires. Although all patients were instructed to complete the questionnaire and reminded by phone during the study, some of them did not record the VAS score or skipped the QOL part for some reason. However, we obtained complete reports for degree of nausea, vomiting, and AE, as planned. Another limitation is that AE were dichotomously self-reported. More quantitative assessments specifically regarding AE, e.g., for sleepiness, should more clearly demonstrate differences in the severity and impact of such adverse events on QOL. Overall, the results of this study can be applied to the majority of oncology practices in Thailand or countries with limited resources.
Conclusions
Given the trend toward better nausea control of OLN10 relative to that provided by APR in patients who received HEC with the similar rate of sleepiness between OLN dosages, addition of OLN10 to ondansetron and dexamethasone is recommended. APR and OLN5 provided similar CINV control, adverse events, and QOL.
References
Ithimakin S, Runglodvatana K, Nimmannit A, Akewanlop C, Srimuninnimit V, Keerativitayanan N, Soparattanapaisarn N, Laocharoenkeat A (2012) Randomized, double-blinded, placebo-controlled trial of ondansetron plus dexamethasone with or without metoclopramide as antiemetic prophylaxis in patients receiving high-dose cisplatin in medical practice. Support Care cancer 20(4):849–855. https://doi.org/10.1007/s00520-011-1162-4
Thamlikitkul L, Srimuninnimit V, Akewanlop C, Ithimakin S, Techawathanawanna S, Korphaisarn K, Chantharasamee J, Danchaivijitr P, Soparattanapaisarn N (2017) Efficacy of ginger for prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adriamycin-cyclophosphamide regimen: a randomized, double-blind, placebo-controlled, crossover study. Support Care Cancer 25(2):459–464. https://doi.org/10.1007/s00520-016-3423-8
Vimolchalao V, Sakdejayont S, Wongchanapai P, Sukprakun S, Angspatt P, Thawinwisan W, Chenaksara P, Sriuranpong V, Vinayanuwatikun C, Parinyanitikun N, Poovorawan N, Tanasanvimon S (2019) The efficacy and safety of the addition of olanzapine to ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. https://doi.org/10.1007/s10147-019-01570-3
Tienchaiananda P, Nipondhkit W, Maneenil K, Sa-Nguansai S, Payapwattanawong S, Laohavinij S, Maneechavakajorn J (2019) A randomized, double-blind, placebo-controlled study evaluating the efficacy of combination olanzapine, ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving doxorubicin plus cyclophosphamide. Ann Palliat Med 8(4):372–380. https://doi.org/10.21037/apm.2019.08.04
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, Dietrich L, Biggs D, Lafky JM, Loprinzi CL (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375(2):134–142. https://doi.org/10.1007/s00520-016-3449-y10.1056/NEJMoa1515725
Yang Y, Fang W, Luo F, Chen X, Ma Y, Zhao Y, Zhan J, Xue C, Hou X, Zhou T, Ma S, Gao F, Huang Y, Chen L, Zhou N, Zhao H, Zhang L, Einhorn LH, Rapoport B, Navari RM, Herrstedt J, Brames MJ (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Oncologist 25(1):303–308. https://doi.org/10.1634/theoncologist.2017-0378
Cheng Y, Shi JH, Chen ZD, Wang QM, Zhang HL, Hu B, Liu B, Zhang QY, Wu Q, Wang D, Shu YQ, Dong J, Han BH, Wang KM, Dang CX, Li JL, Wang HB, Li BL, Lu JG, Zhang ZH, Chen YX, Roila F, Ruggeri B, Ballatori E, Fatigoni S, Caserta C, Licitra L, Mirabile A, Ionta MT, Massidda B, Cavanna L, Palladino MA, Tocci A, Fava S, Colantonio I, Angelelli L, Ciuffreda L, Fasola G, Zerilli F (2015) Aprepitant versus metoclopramide, both combined with dexamethasone, for the prevention of cisplatin-induced delayed emesis: a randomized, double-blind study. Eur J Cancer Care 26(6):1248–1253. https://doi.org/10.1111/ecc.1266810.1093/annonc/mdv132
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol 21(22):4112–4119. https://doi.org/10.1200/jco.2003.01.095
Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Bohidar N, Klinger G, Hustad CM, Horgan KJ, Skobieranda F (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23(12):2822–2830. https://doi.org/10.1200/jco.2005.09.050
Clemmons AB, Orr J, Andrick B, Gandhi A, Sportes C, DeRemer D (2018) Randomized, placebo-controlled, phase III trial of fosaprepitant, ondansetron, dexamethasone (FOND) versus FOND plus olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: the FOND-O Trial. Biol Blood Marrow Transplant 24(10):2065–2071. https://doi.org/10.1016/j.bbmt.2018.06.005
Hashimoto H, Abe M, Yanai T, Yamaguchi T, Zenda S, Uchitomi Y, Fukuda H, Mori M, Iwasa S, Yamamoto N, Ohe Y (2018) Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double blind, placebo-controlled phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol 48(10):950–952. https://doi.org/10.1093/jjco/hyy114
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9(5):188–195. https://doi.org/10.1016/j.suponc.2011.05.002
Zhang Z, Zhang Y, Chen G (2018) Olanzapine-based triple regimens versus neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy: a network meta-analysis. Oncologist 23(5):603–616. https://doi.org/10.1634/theoncologist.2017-0378
Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C (2003) Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer 11(8):522–527. https://doi.org/10.1007/s00520-003-0482-4
Shivaprakash G, Udupa KS, Sarayu V, Thomas J, Gupta V, Pallavi LC, Pemminati S (2017) Olanzapine versus aprepitant for the prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving doxorubicin-cyclophosphamide regimen: a prospective, nonrandomized, open-label study. Indian J Pharmacol 49(6):451–457. https://doi.org/10.4103/ijp.IJP_846_16
Chanthawong S, Lim YH, Subongkot S, Chan A, Andalusia R, Ahmad Bustamam RS, Chaiyakunapruk N (2019) Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for managing highly emetogenic chemotherapy in Southeast Asia: a multinational study. Support Care Cancer 27(3):1109–1119. https://doi.org/10.1007/s00520-018-4400-1
Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N (2018) A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 23(2):382–388. https://doi.org/10.1007/s10147-017-1200-4
Acknowledgments
We thank the pharmacists and nurse’s team at the infusion unit and oncology inpatient ward, Siriraj Hospital, for facilitating patient recruitment and data collection.
Funding
This study was supported by a grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Grant Number (IO) R016231016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial protocol was approved by the Siriraj Institutional Review Board. All recruited patients provided written inform consent. The authors have full control of all primary data and allow the journal to review the data if requested.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ithimakin, S., Theeratrakul, P., Laocharoenkiat, A. et al. Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Support Care Cancer 28, 5335–5342 (2020). https://doi.org/10.1007/s00520-020-05380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05380-6