Abstract
Purpose
To update the 2013 Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) clinical practice guidelines on oral cryotherapy for the management of oral mucositis (OM) caused by cancer therapies.
Methods
A systematic review was conducted by the Mucositis Study Group of MASCC/ISOO. The evidence for each intervention for specific cancer treatment modalities was assigned a level of evidence (LoE). The findings were added to the database used to develop the 2013 MASCC/ISOO clinical practice guidelines. Based on the LoE, the guidelines were set as: recommendation, suggestion, or no guideline possible.
Results
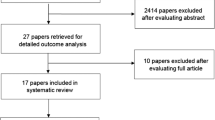
A total of 114 papers were identified: 44 from PubMed and 70 from Web of Science. After abstract triage and merging with the 2013 database, 36 papers were reviewed. The LoE for prevention of OM with oral cryotherapy in patients undergoing autologous hematopoietic stem cell transplant using high-dose melphalan conditioning protocols was upgraded, and the guideline changed to recommendation. Additionally, the recommendation for prevention of OM with oral cryotherapy in patients receiving bolus 5-fluorouracil for the treatment of solid tumors was confirmed. No guidelines were possible for other clinical settings.
Conclusions
The evidence supports recommendations for the use of oral cryotherapy for the prevention of OM for either (i) patients undergoing autologous hematopoietic stem cell transplant with high-dose melphalan conditioning protocols or (ii) patients receiving bolus 5-fluorouracil chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) is a significant toxicity of systemic chemotherapy (CT) and/or radiotherapy (RT) to the head and neck region. It occurs in 20–40% of patients receiving conventional CT, 80% of patients receiving high-dose CT as conditioning for hematopoietic stem cell transplantation (HSCT), and nearly all patients receiving head and neck RT [1]. OM increases morbidity and has a devastating impact on quality of life [2]. Clinically, lesions of OM are atrophic, erythematous, and/or ulcerative. Ulcerative lesions are associated with severe pain, increased risk of severe infections, oral bleeding, compromised oral function and risk of hospitalization [3]. Numerous therapies have been reported for the management of OM [4, 5], including oral cryotherapy. Application of ice or cold water in the oral cavity is the conventional method of administrating cryotherapy, and new technologies generating a cool environment in the mouth have also been reported [6]. It is hypothesized that the vasoconstriction caused by the ice restricts the delivery of cytotoxic drugs to the oral tissues and thereby reduces secondary complications [7, 8]. In addition, it was suggested that reduced tissue temperature lowered the metabolic activity in the basal layer, rendering the epithelium less susceptible to cytotoxic agents [9, 10].
In 2004, the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) published the first guidelines addressing the use of oral cryotherapy for the management of OM [11], which remained unchanged in the 2007 and in 2013 updates [4, 12, 13]. Considering the findings published since the last update, MASCC/ISOO initiated a 3rd guideline update to systematically evaluate the peer-reviewed literature published after 2011 and update the MASCC/ISOO clinical practice guidelines for the management of OM with oral cryotherapy.
Methods
The methods are described in detail by Ranna et al. [14]. Briefly, a search for relevant papers indexed in the literature from 1 January 2011 to 30 June 2016 was conducted using PubMed/Web of Science, with papers selected for reviews based on specific inclusion and exclusion criteria [14]. Literature that was published following the end date of the literature search is covered in the “Discussion” as “Late breaking news”.
Publications were reviewed by two independent reviewers and data were extracted using a standard electronic form. Studies were scored for their level of evidence (LoE) based on criteria from Somerfield et al. [15], and flaws were listed according to the Hadorn criteria [16]. A well-designed study was defined as a study with no major flaws according to the Hadorn criteria.
Findings from the studies reviewed were merged with the evidence in the previous MASCC/ISOO guideline update and then integrated into the new guidelines based on the overall LoE for each intervention. Guidelines were classified as Recommendation, Suggestion, and No Guideline Possible.
Guidelines were organized according to: (1) the aim of the intervention (prevention or treatment of OM), (2) the treatment modality (RT, CT, RT–CT, or high-dose conditioning therapy for HSCT), and (3) the route of administration of the intervention.
The list of keywords used for the literature search for the cryotherapy section is in the published paper with the methodology paper [14].
Results
A total of 114 papers were identified in the literature search: 44 from PubMed and 70 from Web of Science. After abstract triage, 15 new papers were included in this review. These papers were merged with the 21 papers reviewed for the previous MASCC/ISOO guidelines update and analyzed collectively [13].
In total, 93% of the oral cryotherapy studies reviewed were directed at prevention and a single study used oral cryotherapy for OM treatment in patients with solid cancer [17]. The results of the review are presented according to patient population categories.
Hematopoietic stem cell transplantation (HSCT) for hematological cancer; for OM prevention
2013 Guideline: Suggestion (LoE III)
2018 Guideline: Recommendation (LoE II)
The panel recommends using oral cryotherapy to prevent oral mucositis in patients undergoing autologous HSCT when the conditioning includes high-dose melphalan (LoE II)
The recommendation is based on two new randomized controlled trials (RCTs) [18, 19]. One RCT demonstrated the efficacy of oral cryotherapy in the prevention of OM and mucositis-associated pain in patients undergoing high-dose melphalan protocols for autologous HSCT [19]. This study compared oral cryotherapy and basic oral care with a control group that only received basic oral care. An additional RCT assessing patients undergoing autologous HSCT with high-dose melphalan-based conditioning regimens showed that oral cryotherapy reduces OM severity compared with a saline mouthwash [18].
These results were added to the results from four RCTs reviewed in the previous guidelines update [13]. The first out of these four papers was a RCT on autologous HSCT patients treated with high-dose melphalan, which reported reduced OM severity [10]. Two studies on autologous and allogeneic HSCT patients, some of which were treated with high-dose melphalan, reported the benefit of oral cryotherapy on the prevention of OM [20, 21]. Since these studies utilized the same patient population, they are considered as a single study for the purpose of this analysis. These results are in conflict with a fourth large RCT in allogeneic HSCT patients [22], which reported no benefit from oral cryotherapy in OM prevention. All patients in the Gori et al. study received methotrexate (for graft versus host disease [GVHD] prophylaxis), and some were also treated with melphalan (for conditioning regimen) [22]. The current guidelines therefore only refer to patients undergoing autologous HSCT conditioned with high-dose melphalan.
Non-RCT studies showed similar results about the usefulness of oral cryotherapy in autologous HSCT with high-dose melphalan protocols [23,24,25,26,27,28,29,30,31,32].
An additional RCT applied oral cryotherapy for the prevention of OM in HSCT patients; however, in this study oral cryotherapy was referred to as a standard of care in a protocol mixing oral cryotherapy and supersaturated calcium phosphate rinses [33]. This RCT showed no differences in the prevention of OM when oral cryotherapy with supersaturated calcium phosphate rinses was compared with oral cryotherapy alone in patients undergoing to allogeneic HSCT with different regimens for hematological cancer [33]. Another before-and-after study applied oral cryotherapy as standard of care while assessing the effect of laser therapy on the prevention of OM in HSCT patients [34]. The experimental group had significantly less severe mucositis.
Chemotherapy with bolus 5-FU; for solid tumors; for OM prevention
2013 guideline: Recommendation (LoE II)
2018 guideline: Recommendation (LoE II)
The panel recommends that patients receiving bolus 5-FU chemotherapy undergo 30 min of oral cryotherapy to prevent oral mucositis.
One new RCT was identified in the literature supporting previous RCTs in this category [35]. This RCT included patients with various types of solid cancer and showed that oral cryotherapy significantly reduced OM severity, especially on days 7 and 14 following bolus 5-FU [35]. This RCT concurs with the results of RCTs reviewed in the previous guidelines update [8, 36,37,38,39,40].
The optimal duration of oral cryotherapy as a preventive measure for OM in patients with various solid cancers using bolus 5-FU-based protocols has been evaluated, and 60 min was not found to be superior to 30 min [41].
Chemotherapy with short-term infusion and short half-life agents; solid cancer; for OM prevention
2013 Guideline: No guideline possible (LoE III)
2018 Guideline: No guideline possible (LoE III)
The efficacy of oral cryotherapy for OM prevention in patients undergoing protocols using drugs over a short-term or with a short half-life, other than 5-FU, has been established.
A RCT study demonstrated the efficacy of oral cryotherapy in cancer patients treated with various agents, such as etoposide, platinum, mitomycin C, and vinblastine [7]. The same beneficial preventive effect was noted in a cohort that included patients treated with edatrexate plus carboplatin [42, 43]. However, in a different cohort study, the preventive effect was not confirmed in patients treated with edatrexate [44].
Therefore, given that the evidence the 2013 publication stated “no guideline possible,” this remains unchanged in the current update as no new studies were identified.
Radiotherapy; head and neck cancer; for OM prevention
2018 Guideline: no guideline possible (LoE III)
A single RCT showed no significant changes in OM severity in patients treated with RT to the head and neck when oral cryotherapy was delivered before and after each RT session. The average RT dose delivered in this study was 53.9 Gy. Importantly, patient perception of mucositis and pain severity were worse in the treatment group [45]. There is insufficient evidence to formulate a guideline.
Chemotherapy; solid cancer; for OM treatment
2018 Guideline: no guideline possible (LoE III)
The efficacy of oral cryotherapy versus “routine care” or chlorhexidine was compared in a RCT that focused on regaining the ability to eat, in patients that could not do so due to OM, termed “oral nutrition transition time.” The study included patients with grade 3–4 OM due to CT, which was not specified [17]. The study did not report the response in terms of mucositis grade or associated pain. The study concluded that oral cryotherapy was not superior to “regular care” or chlorhexidine. Since the type of CT is critical to developing a guideline regarding use of oral cryotherapy, this evidence is insufficient to determine a guideline.
Chemotherapy with 5-FU (continuous); solid cancer; for OM prevention
2013 Guideline: no guideline possible (LoE III)
2018 Guideline: no guideline possible (LoE III)
There is no new published evidence regarding oral cryotherapy for prevention of OM due to continuous infusion of 5-FU following publication of the 2013 MASCC/ISOO guidelines, understanding that it would not be predicted to work in this situation.
Discussion
This paper reports results of a systematic review of evidence about oral cryotherapy for OM in cancer patients, and therefore updates the MASCC/ISOO clinical practice guidelines for the management of OM [13] as needed. Evidence supporting clinical practice guidelines was identified for two indications:
(i)Oral cryotherapy is recommended for the prevention of OM in patients undergoing autologous HSCT with conditioning regimen protocols, including high-dose melphalan. The current systematic review added two RCTs that enhanced the LoE from III to II, which strengthens the 2013 guidelines from a suggestion to a recommendation [18, 19].
(ii)Oral cryotherapy is recommended for the prevention of OM in patients receiving bolus 5-FU for solid tumors when used during the 30-min CT infusion. A new RCT supported this [35].
Oral cryotherapy for the prevention of RT-associated OM was first reported in 2013 [45]. In this study, oral cryotherapy was delivered 5 min before and after each RT session. The patients experienced significantly less OM pain and symptoms [45]. Due to the limited data, no guideline is possible in this category.
two categories from the 2013 guideline update combined in this review because they described OM associated with CT delivered over a short time, excluding 5-FU, specifically “CT (edatrexate i.v.) (Investigational drug)” and “CT (etoposide, mitomycin C, vinblastine i.v.)” and are referred to in this 2019 update as “CT infused over a short time or drugs with short-half life, other than 5-FU”. The rationale for merging the two categories was that the critical parameter for the effect of oral cryotherapy was the short duration of the infusion rather than cytotoxic agent itself.
As cited in the 2013 guidelines, edatrexate has not progressed from an investigational drug to a registered drug. No new studies were published in this category; therefore, guideline remains unchanged—no guideline possible. Having said this, the rationale for oral cryotherapy working in this situation is similar to the rationale as to why it might work for bolus 5-FU therapy. In addition, the studies did support the concept that it was helpful in this situation. Thus, while it does not make the mark to be guideline-endorsed, use of this approach does make rational clinical sense.
Two meta-analyses addressing oral cryotherapy for OM had similar conclusions to the current guidelines. One addressed the efficacy of oral cryotherapy in OM prevention in patients with hematological malignancies undergoing HSCT [46] based on seven RCTs, with two published as abstracts only. This meta-analysis concluded that oral cryotherapy significantly decreased OM severity and reduced the duration of total parenteral nutrition and the length of hospitalization. Their results regarding OM duration and analgesic use were non-significant with a trend toward a reduction in the length of analgesic use [46]. A Cochrane meta-analysis supported these findings, which included 14 RCTs on oral cryotherapy in cancer patients [47]. The Cochrane meta-analysis concluded that oral cryotherapy was effective in two conditions: (1) after receiving 5-FU-based treatment for solid tumors and (2) after receiving high-dose melphalan-based cancer treatment prior to HSCT. For the latter indication, there was uncertainty regarding the extent of OM reduction because of the large confidence interval (2–111) for the parameter of number of patients needed to be treated (3). The Cochrane review also evaluated some secondary outcome measures, interruption to cancer treatment, oral pain, quality of life, normalcy of diet (e.g., percutaneous endoscopic gastrostomy feeding tubes or total parenteral nutrition), adverse events, number of days in hospital, number of days of treatment with opioid analgesics, and number of days unable to take medicine orally. However, data were limited, or the assessments of these outcomes were not performed.
Oral cryotherapy is an easily applied affordable treatment modality for OM management. While no serious adverse effects have been reported for oral cryotherapy [47], when flavored popsicles have been used, more adverse effects were reported, including nausea, oral sensitivity, and headaches [38].
There is some variability between duration of oral cryotherapy in the RCTs reported in HSCT patients conditioned with melphalan. A recent RCT compared two groups of HSCT patients conditioned with melphalan treated with oral cryotherapy for either 2 h or 6 h [48]. The shorter oral cryotherapy regimen was at least as efficacious as the 6-h protocol [10]. A similar study design was conducted by another group comparing 2-h oral cryotherapy with 7-h oral cryotherapy patients undergoing autologous HSCT [49]. This study found no difference between the arms and therefore concluded that the 2-h oral cryotherapy is as effective as 7-h oral cryotherapy in preventing OM in this clinical setting. Another RCT in patients undergoing HSCT with melphalan-based conditioning used a protocol starting 5 min before cytotoxic conditioning and ending 5 min after cytotoxic conditioning with a break in the oral cryotherapy up to 20 min during this time [18]. An earlier RCT in patients undergoing HSCT reported that oral cryotherapy was applied for a total of 60 min starting before cytotoxic conditioning and ending after cytotoxic conditioning completion [19]. Of note, in non-RCT studies in patients undergoing HSCT, shorter oral cryotherapy protocols were reported [24, 29, 32], or longer protocols [23, 27].
As a general statement, the lack of sham treatment and patient blindness to the treatment may affect the results of oral cryotherapy trials. However, this is a justifiable study design flaw, as it is not feasible to blind patients to the temperature of the treatment.
The Pediatric Oncology Group of Ontario published guidelines for OM prevention in pediatric cancer patients [50]. The use of oral cryotherapy was recommended for cooperative children receiving CT or HSCT conditioning regimens with short infusion or short half-life times; however, no clinical study in pediatric patients was available. Considering that oral cryotherapy can be delivered using flavored ice popsicles and ice slushy drinks—“freezies” or “smoothies”—compliance in children is expected to be good.
As “late breaking news”, two RCTs were published after the cut-off date of the literature search addressing oral cryotherapy for prevention of OM. One concurred with the findings of these current guidelines, showing that oral cryotherapy is effective in patients receiving high-dose melphalan conditioning regimen for autologous HSCT [51]. The other confirmed our guidelines in cancer patients treated with bolus 5-FU showing that oral cryotherapy was effective in reducing mucositis severity and associated pain [52].
Interestingly, a new patient population treated with oral cryotherapy was reported in the Chinese literature: osteosarcoma patients treated with high-dose methotrexate [53]. Although this study was not within the scope of our systematic review, it might open the door for additional research in this patient population. Of relevance, low-dose methotrexate-associated OM was studied in a RCT [22] and in a comparative study [32] and was included in our systematic review.
Based upon the literature, it appears that oral cryotherapy has become standard of care in certain clinical settings and that it is practiced across many oncology programs at the international level. It is encouraging that a relatively simple intervention became a consensus for the prevention of OM and is applied in numerous institutes. Actually, certain studies testing new interventions for OM had to apply it in combination with oral cryotherapy in order not to deviate from the standard of care [54, 55]. This approach may add new challenges, as it is unclear if the additive effect of new intervention is maintained in a cold environment.
On a related matter, oral cryotherapy was suggested to be an independent suppressive factor for dysgeusia in patients undergoing autologous HSCT [56]. CT-induced dysgeusia is one of the reasons for reduced oral intake in cancer patients. These results mean that oral cryotherapy may maintain the oral intake not just by preventing OM but also by preventing dysgeusia. This indication for oral cryotherapy warrants additional research.
In summary, the MASCC/ISOO guideline update recommends oral cryotherapy for the prevention of OM for patients undergoing autologous HSCT with high-dose melphalan conditioning protocols and for patients receiving treatment for solid cancer with 5-FU.
References
Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS (2006) Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 14:505–515
McGuire DB, Fulton JS, Park J, Brown CG, Correa ME, Eilers J, Elad S, Gibson F, Oberle-Edwards LK, Bowen J, Lalla RV, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral O (2013) Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer 21:3165–3177
Lalla RV, Saunders DP, Peterson DE (2014) Chemotherapy or radiation-induced oral mucositis. Dent Clin N Am 58:341–349
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S, Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in C, International Society of Oral O (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Villa A, Sonis S (2016) Toxicities associated with head and neck cancer treatment and oncology-related clinical trials. Curr Probl Cancer 40:244–257
Walladbegi J, Gellerstedt M, Svanberg A, Jontell M (2017) Innovative intraoral cooling device better tolerated and equally effective as ice cooling. Cancer Chemother Pharmacol 80:965–972
Karagozoglu S, Filiz Ulusoy M (2005) Chemotherapy: the effect of oral cryotherapy on the development of mucositis. J Clin Nurs 14:754–765
Mahood DJ, Dose AM, Loprinzi CL, Veeder MH, Athmann LM, Therneau TM, Sorensen JM, Gainey DK, Mailliard JA, Gusa NL et al (1991) Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol 9:449–452
Walladbegi J, Smith SA, Grayson AK, Murdoch C, Jontell M, Colley HE (2018) Cooling of the oral mucosa to prevent adverse effects of chemotherapeutic agents: an in vitro study. J Oral Pathol Med 47:477–483
Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, Maloney DG, Press OW, Bensinger W (2006) A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 37:1031–1035
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST, Mucositis Study Section of the Multinational Association for Supportive Care in C, International Society for Oral O (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100:2026–2046
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE, Mucositis Study Section of the Multinational Association of Supportive Care in C, the International Society for Oral O (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Peterson DE, Ohrn K, Bowen J, Fliedner M, Lees J, Loprinzi C, Mori T, Osaguona A, Weikel DS, Elad S, Lalla RV, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral O (2013) Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 21:327–332
Ranna VC, K ; Castillo, D; Porcello, L; Vaddi, A; Lalla, R.; Bossi, P; Elad, S (2019) Development of the MASCC/ISOO clinical practice guidelines for Mucositis: an overview of the methods. Jounal Supportive Care in Cancer (In Press)
Somerfield MR, Pfister DG, Bennett CL, Recht A, Smith TJ, Weeks JC, Winn RJ, Durant JR (2000) ASCO clinical practice guidelines: process. Progress, Pitfalls, and Prospects Classic Papers and Current Comments 4:881–886
Hadorn DC, Baker D, Hodges JS, Hicks N (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49:749–754
Erden Y, Ipekcoban G (2017) Comparison of efficacy of cryotherapy and chlorhexidine to oral nutrition transition time in chemotherapy-induced oral mucositis. Eur J Cancer Care (Engl) 26(5). https://doi.org/10.1111/ecc.12495 Epub 2016 May 1
Askarifar M, Lakdizaji S, Ramzi M, Rahmani A, Jabbarzadeh F (2016) The effects of oral cryotherapy on chemotherapy-induced oral mucositis in patients undergoing autologous transplantation of blood stem cells: a clinical trial. Iran Red Crescent Med J 18:e24775
Salvador P, Azusano C, Wang L, Howell D (2012) A pilot randomized controlled trial of an oral care intervention to reduce mucositis severity in stem cell transplant patients. J Pain Symptom Manag 44:64–73
Svanberg A, Birgegard G, Ohrn K (2007) Oral cryotherapy reduces mucositis and opioid use after myeloablative therapy--a randomized controlled trial. Support Care Cancer 15:1155–1161
Svanberg A, Ohrn K, Birgegard G (2010) Oral cryotherapy reduces mucositis and improves nutrition-a randomised controlled trial. J Clin Nurs 19:2146–2151
Gori E, Arpinati M, Bonifazi F, Errico A, Mega A, Alberani F, Sabbi V, Costazza G, Leanza S, Borrelli C, Berni M, Feraut C, Polato E, Altieri MC, Pirola E, Loddo MC, Banfi M, Barzetti L, Calza S, Brignoli C, Bandini G, De Vivo A, Bosi A, Baccarani M (2007) Cryotherapy in the prevention of oral mucositis in patients receiving low-dose methotrexate following myeloablative allogeneic stem cell transplantation: a prospective randomized study of the Gruppo Italiano Trapianto di Midollo Osseo nurses group. Bone Marrow Transplant 39:347–352
Aisa Y, Mori T, Kudo M, Yashima T, Kondo S, Yokoyama A, Ikeda Y, Okamoto S (2005) Oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer 13:266–269
Batlle M, Morgades M, Vives S, Ferra C, Oriol A, Sancho JM, Xicoy B, Moreno M, Magallon L, Ribera JM (2014) Usefulness and safety of oral cryotherapy in the prevention of oral mucositis after conditioning regimens with high-dose melphalan for autologous stem cell transplantation for lymphoma and myeloma. Eur J Haematol 93:487–491
Bhatt V, Vendrell N, Nau K, Crumb D, Roy V (2010) Implementation of a standardized protocol for prevention and management of oral mucositis in patients undergoing hematopoietic cell transplantation. J Oncol Pharm Pract 16:195–204
Chen J, Seabrook J, Fulford A, Rajakumar I (2017) Icing oral mucositis: oral cryotherapy in multiple myeloma patients undergoing autologous hematopoietic stem cell transplant. J Oncol Pharm Pract 23:116–120
Mori T, Aisa Y, Yamazaki R, Mihara A, Ikeda Y, Okamoto S (2006) Cryotherapy for the prevention of high-dose melphalan-induced oral mucositis. Bone Marrow Transplant 38:637–638
Ohbayashi Y, Imataki O, Ohnishi H, Iwasaki A, Ogawa T, Inagaki N, Shigeta H, Ohue Y, Tasaka T, Kitanaka A, Kubota Y, Tanaka T, Ishida T, Miyake M (2008) Multivariate analysis of factors influencing oral mucositis in allogeneic hematopoietic stem cell transplantation. Ann Hematol 87:837–845
Sato A, Saisho-Hattori T, Koizumi Y, Minegishi M, Iinuma K, Imaizumi M (2006) Prophylaxis of mucosal toxicity by oral propantheline and cryotherapy in children with malignancies undergoing myeloablative chemo-radiotherapy. Tohoku J Exp Med 210:315–320
Vokurka S, Bystricka E, Scudlova J, Mazur E, Visokaiova M, Vasilieva E, Brandejsova R, Chvojkova I, Vrabcova M, Vitkova J, Mjartanova D, Vodickova M, Bockova J, Streinerova K (2011) The risk factors for oral mucositis and the effect of cryotherapy in patients after the BEAM and HD-l-PAM 200 mg/m(2) autologous hematopoietic stem cell transplantation. Eur J Oncol Nurs 15:508–512
Vokurka S, Chvojkova I, Svoboda T, Brandejsova R, Jungova A, Bystricka E, Jindra P (2014) The impact of oral cryotherapy and oral and gastrointestinal mucositis after autologous stem cell transplantation. Eur J Oncol Nurs 18:228–229
Vokurka S, Svoboda T, Jungova A, Karas M, Koza V (2012) Oral cryotherapy can significantly reduce oral mucositis but not acute GVHD incidence in Flu/Mel conditioning allo-SCT. Bone Marrow Transplant 47:739–741
Svanberg A, Ohrn K, Birgegard G (2015) Caphosol((R)) mouthwash gives no additional protection against oral mucositis compared to cryotherapy alone in stem cell transplantation. A pilot study. Eur J Oncol Nurs 19:50–53
de Paula EF, Bezinelli LM, da Graca Lopes RM, Nascimento Sobrinho JJ, Hamerschlak N, Correa L (2015) Efficacy of cryotherapy associated with laser therapy for decreasing severity of melphalan-induced oral mucositis during hematological stem-cell transplantation: a prospective clinical study. Hematol Oncol 33:152–158
Katranci N, Ovayolu N, Ovayolu O, Sevinc A (2012) Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy-a randomized controlled trial. Eur J Oncol Nurs 16:339–344
Baydar M, Dikilitas M, Sevinc A, Aydogdu I (2005) Prevention of oral mucositis due to 5-fluorouracil treatment with oral cryotherapy. J Natl Med Assoc 97:1161–1164
Cascinu S, Fedeli A, Fedeli SL, Catalano G (1994) Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur J Cancer B Oral Oncol 30B:234–236
Nikoletti S, Hyde S, Shaw T, Myers H, Kristjanson LJ (2005) Comparison of plain ice and flavoured ice for preventing oral mucositis associated with the use of 5 fluorouracil. J Clin Nurs 14:750–753
Papadeas E, Naxakis S, Riga M, Kalofonos C (2007) Prevention of 5-fluorouracil-related stomatitis by oral cryotherapy: a randomized controlled study. Eur J Oncol Nurs 11:60–65
Sorensen JB, Skovsgaard T, Bork E, Damstrup L, Ingeberg S (2008) Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 112:1600–1606
Rocke LK, Loprinzi CL, Lee JK, Kunselman SJ, Iverson RK, Finck G, Lifsey D, Glaw KC, Stevens BA, Hatfield AK et al (1993) A randomized clinical trial of two different durations of oral cryotherapy for prevention of 5-fluorouracil-related stomatitis. Cancer 72:2234–2238
Edelman MJ, Gandara DR, Perez EA, Lau D, Lauder I, Turrell C, Uhrich M, Meyers F (1998) Phase I trial of edatrexate plus carboplatin in advanced solid tumors: amelioration of dose-limiting mucositis by ice chip cryotherapy. Investig New Drugs 16:69–75
Gandara DR, Edelman MJ, Crowley JJ, Lau DH, Livingston RB (1997) Phase II trial of edatrexate plus carboplatin in metastatic non-small-cell lung cancer: a Southwest Oncology Group study. Cancer Chemother Pharmacol 41:75–78
Dreicer R, Propert KJ, Kuzel T, Kirkwood JM, O'Dwyer PJ, Loehrer PJ (1997) A phase II trial of edatrexate in patients with advanced renal cell carcinoma. An Eastern Cooperative Oncology Group study. Am J Clin Oncol 20:251–253
Kakoei SG, A., Nakhaee, N (2013) Effect of cryotherapy on oral mucositis in patients with head and neck cancers receiving radiotherapy. Int J Radiat Res 11: 117–120
Wang L, Gu Z, Zhai R, Zhao S, Luo L, Li D, Zhao X, Wei H, Pang Z, Wang L, Liu D, Wang Q, Gao C (2015) Efficacy of oral cryotherapy on oral mucositis prevention in patients with hematological malignancies undergoing hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. PLoS One 10:e0128763
Riley P, Glenny AM, Worthington HV, Littlewood A, Clarkson JE, McCabe MG (2015) Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy. Cochrane Database Syst Rev: CD011552
Cho YK, Sborov DW, Lamprecht M, Li J, Wang J, Hade EM, Gao Y, Tackett K, Williams N, Benson DM, Efebera YA, Rosko AE, Devine SM, Poi M, Hofmeister CC, Phelps MA (2017) Associations of high-dose melphalan pharmacokinetics and outcomes in the setting of a randomized cryotherapy trial. Clin Pharmacol Ther 102:511–519
Johansson JE, Bratel J, Hardling M, Heikki L, Mellqvist UH, Hasseus B (2019) Cryotherapy as prophylaxis against oral mucositis after high-dose melphalan and autologous stem cell transplantation for myeloma: a randomised, open-label, phase 3, non-inferiority trial. Bone Marrow Transplant 54(9):1482–1488. https://doi.org/10.1038/s41409-019-0468-6
Sung L, Robinson P, Treister N, Baggott T, Gibson P, Tissing W, Wiernikowski J, Brinklow J, Dupuis LL (2017) Guideline for the prevention of oral and oropharyngeal mucositis in children receiving treatment for cancer or undergoing haematopoietic stem cell transplantation. BMJ Support Palliat Care 7:7–16
Marchesi F, Tendas A, Giannarelli D, Viggiani C, Gumenyuk S, Renzi D, Franceschini L, Caffarella G, Rizzo M, Palombi F, Pisani F, Romano A, Spadea A, Papa E, Canfora M, Pignatelli A, Cantonetti M, Arcese W, Mengarelli A (2017) Cryotherapy reduces oral mucositis and febrile episodes in myeloma patients treated with high-dose melphalan and autologous stem cell transplant: a prospective, randomized study. Bone Marrow Transplant 52:154–156
Idayu Mat Nawi R, Lei Chui P, Wan Ishak WZ, Hsien Chan CM (2018) Oral cryotherapy: prevention of oral mucositis and pain among patients with colorectal cancer undergoing chemotherapy. Clin J Oncol Nurs 22:555–560
Zhang WLL, Lu YW, Zhen JC, Zhang XL (2011) Intervention research on preventing oral mucositis after using high dose methotrexate chemotherapy in osteosarcoma by gargling with calcium folinic. Chinese Phamaceutical Journal 14:1126–1128
Dos Reis PE, Ciol MA, de Melo NS, Figueiredo PT, Leite AF, Manzi Nde M (2016) Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: a pilot study. Support Care Cancer 24:4393–4398
Yokomizo H, Yoshimatsu K, Hashimoto M, Ishibashi K, Umehara A, Yoshida K, Fujimoto T, Watanabe K, Ogawa K (2004) Prophylactic efficacy of allopurinol ice ball for leucovorin/5-fluorouracil therapy-induced stomatitis. Anticancer Res 24:1131–1134
Okada N, Hanafusa T, Abe S, Sato C, Nakamura T, Teraoka K, Abe M, Kawazoe K, Ishizawa K (2016) Evaluation of the risk factors associated with high-dose chemotherapy-induced dysgeusia in patients undergoing autologous hematopoietic stem cell transplantation: possible usefulness of cryotherapy in dysgeusia prevention. Support Care Cancer 24:3979–3985
Dumontet C, Sonnet A, Bastion Y, Salles G, Espinouse D, Coiffier B (1994) Prevention of high dose L-PAM-induced mucositis by cryotherapy. Bone Marrow Transplant 14:492–494
Farrington M, Cullen L, Dawson C (2014) Cryotherapy is a simple nursing intervention for oral mucositis. ORL Head Neck Nurs 32:13–15
Acknowledgments
The authors would like to thank the following medical librarians for their valuable contribution to this project:
Lorraine Porcello, MSLIS, MSIM—Bibby Dental Library, Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, NY, USA and Daniel A. Castillo, MLIS—Edward G. Miner Library, University of Rochester Medical Center, Rochester, NY, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Per the MASCC Guidelines Policy, employees of commercial entities were not eligible to serve on this MASCC Guidelines Panel. All authors completed a Conflict of Interest disclosure form and conflicts are disclosed in the guideline’s publications.
The authors disclose no conflict of interest (MEPC, KC, AK, TM, CP, TR, JJT, VR, AV, DEP). PB has served an advisory role for Galera Therapeutics, AstraZeneca, Helsinn, and Kyowa Kyrin and received grants from Merck, Kyowa Kyrin, and Roche.
CLL reports personal fees from PledPharma, Disarm Therapeutics, Asahi Kasei, Metys Pharmaceuticals, and OnQuality, outside the submitted work.
RVL reports personal fees from Colgate Oral Pharmaceuticals, grants and personal fees from Galera Therapeutics, personal fees from Ingalfarma, other from Logic Biosciences, personal fees from Monopar Therapeutics, personal fees from Mundipharma, grants from Novartis, grants from Oragenics, grants and personal fees from Sucampo Pharma, personal fees from Alira Health, outside the submitted work.
SE reports no conflict in regard to mucositis and consulting to Falk Pharma outside the submitted work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO)
Rights and permissions
About this article
Cite this article
Correa, M.E.P., Cheng, K.K.F., Chiang, K. et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 28, 2449–2456 (2020). https://doi.org/10.1007/s00520-019-05217-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05217-x