Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) may necessitate chemotherapy dose reduction, delay, or discontinuation. This pilot study tested feasibility of patient enrollment, CIPN screening, and data collection in cancer patients for a future clinical study that will assess the safety and efficacy of an intervention that may prevent CIPN.
Methods
This prospective, observational, single-center, pilot study included adults with newly diagnosed lymphoma or multiple myeloma receiving neurotoxic chemotherapy. Patients were enrolled between September 2016 and February 2017. The Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) questionnaire was completed by patients at 3 time points: baseline, week 6, and week 12. The primary outcome was change in the neurotoxicity score between these time points.
Results
Of 33 patients approached for consent, 28 (85%) provided consent and were enrolled. The FACT/GOG-Ntx questionnaire was completed by 28 (100%) at baseline, 25 (89%) at week 6, and 24 (86%) at week 12. Average (standard deviation) neurotoxicity scores were 36.5 (6.6) at baseline, 34.0 (8.3) at week 6, and 30.6 (7.6) at week 12. Neurotoxicity scores changed from baseline by − 2.7 points (95% CI − 5.5 to 0.1; p = 0.061) at week 6 and − 6.0 points (95% CI − 5.6 to − 0.8; p = 0.012) at week 12. Clinically meaningful declines (decrease of > 10% from baseline) in neurotoxicity score were detected in 36% (9 of 25) at week 6 and in 67% (16 of 24) at week 12.
Conclusion
Sixty-seven percent of patients experienced clinically significant CIPN within 12 weeks of starting chemotherapy. Feasibility metrics for enrollment, consent, CIPN assessment, and follow-up were met.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chemotherapy-induced peripheral neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN) is a disabling and dose limiting treatment–related adverse effect usually occurring in a “stocking-glove” distribution [1, 2]. Chemotherapeutic agents—such as carfilzomib, ixazomib, lenalidomide, and pomalidomide—can damage sensory, motor, or autonomous nerve fibers. The time to onset of damage varies with each agent, increasing in a cumulative pattern with each dose [2,3,4]. Symptoms of sensory neuropathy, the most common form of CIPN, range from numbness in the extremities, loss of proprioception, paresthesia, hyperalgesia, to allodynia [4, 5]. Symptoms of motor neuropathy include muscle weakness, cramping, or myoclonus and those of autonomic neuropathy include orthostatic hypotension, severe constipation, and erectile dysfunction [5]. Use of neurotoxic agents, smoking, excessive alcohol use, diabetes mellitus, vitamin deficiencies (vitamin B1, B6, pantothenic acid, alpha-tocopherol, folate, or B12), African American race, older age, autoimmune disease, and genetic factors are some of the factors associated with a risk of CIPN [2, 3, 5].
Approximately 30 to 40% of patients using neurotoxic chemotherapy develop peripheral neuropathy and this adds to their annual healthcare cost [6]. Although usually not life threatening, CIPN interferes with optimal treatment of active disease due to potential dose reductions, treatment delays, and premature cessation of chemotherapy [5, 7]. CIPN decreases the patient’s quality of life (QOL) and negatively impacts activities of daily living among cancer survivors and persists for months to years beyond chemotherapy completion [7, 8]. Although CIPN already occurs commonly, the prevalence of CIPN could increase in the future if new treatment protocols use more intensive dosing or combinations of neurotoxic chemotherapy agents or if cancer patients who were treated with neurotoxic chemotherapy experience prolonged survival [9]. Thus, studies need to look at measures to improve the quality of life of patients living with cancer, the measure targeted in this study being CIPN [10,11,12].
Multiple myeloma and lymphoma
Lymphoma and multiple myeloma are the two most common forms of hematologic cancers in the USA [13,14,15,16,17]. Multiple myeloma and lymphoma have a unique relation with peripheral neuropathy; CIPN in patients with these malignancies is not only associated with the neurotoxic chemotherapy (bortezomib, vincristine, thalidomide) used to treat the cancer but also with the disease itself [18,19,20,21,22,23].
Measuring CIPN
CIPN can be evaluated by subjective (patient-reported outcomes and physician grading) and objective (such as clinical or neurophysiological) measures [24]. One major challenge with the accurate assessment and quantification of CIPN is that there is currently no standardized approach that is consistently or precisely implemented [3]. Currently CIPN can be diagnosed using physician-based instruments, patient-based assessment, or quantitative sensory tests (QST) [3, 25]. The Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) tool is a comprehensive questionnaire developed in 1998 by the Gynecologic Oncology Group in collaboration with the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System [26]. This tool was devised to assess the patients’ perception of the severity and symptoms associated with chemotherapy-induced neuropathy [27].
Treatment and prevention of CIPN
Multiple pharmacologic agents including prescription drugs and over-the-counter products have been studied for the prevention and treatment of peripheral neuropathy [25, 28,29,30]. The American Society of Clinical Oncology (ASCO) published a practice guideline in 2015 after performing a systematic literature review of 48 RCTs on the prevention and treatment of CIPN [31]. Based on this study, ASCO concluded that none of the 19 agents in 42 RCTs have proven to be clinically significant in preventing CIPN [31]. Six RCTs studied the treatment of existent CIPN and based on efficacy information from a large placebo-controlled RCT, there was a moderate recommendation supporting the use of duloxetine in clinical practice to treat painful CIPN [31]. Although there are no evidence-based, consensus approaches to prevent or treat CIPN, anecdotal experience suggests that a combination over-the-counter (OTC) supplements, including alpha lipoic acid 600 mg once daily, thiamine (vitamin B1) 100 mg once daily, and pyridoxine (vitamin B6) 50 mg once daily, may prevent CIPN severity. These agents have not been studied in the 48 RCTs included in the systematic literature review published by ASCO [31]. Alpha lipoic acid is an antioxidant proven to decrease peripheral neuropathy in diabetic patients [29, 30]. Thiamine and pyridoxine deficiencies have been associated with neuropathy in diabetic patients. Dietary supplementation of these vitamins has been shown to decrease painful neuropathy in diabetic patients [28].
Purpose of this study
The purpose of this study is to generate preliminary data on incidence and symptomatic development of CIPN in patients at a tertiary academic medical institution using the FACT/GOG-Ntx questionnaire at three time points: baseline (defined as the start of chemotherapy), week 6, and week 12. This pilot study is designed to test the feasibility of patient enrollment, CIPN screening, and data collection in cancer patients for a future clinical study that will assess the safety and efficacy of an intervention that may prevent CIPN. Since there is a paucity of clinical data assessing the efficacy of combination OTC supplements in preventing or treating peripheral neuropathy in patients with multiple myeloma and lymphoma, this study will report the distribution of FACT/GOG-Ntx among patients who did and did not receive the combination of alpha lipoic acid, thiamine, and pyridoxine as a prespecified subgroup analysis.
Methods
Study site and participants
This study was conducted at the Houston Methodist Hospital, a tertiary academic medical center located in the Texas Medical Center, Houston, Texas. It was approved by the Institutional Review Board and the patients participating in this study provided written informed consent. Patients 18 years or older with a diagnosis of lymphoma or multiple myeloma scheduled to receive bortezomib, brentuximab, carfilzomib, ixazomib, lenalidomide, pomalidomide, vinblastine, or vincristine either in the inpatient or outpatient settings were eligible for the study. Eligible patients were enrolled within 2 weeks of first treatment with these medications. Patients were excluded due to the inability to take oral medications, pregnancy, or if investigators were not able to obtain informed consent. Patients were excluded from the study if they withdrew consent. Those unable to complete follow-up assessments either in person or over the phone were considered as lost to follow-up.
Study design
This prospective, single-center, pilot, cohort study screened and enrolled patients over a 6-month period (September 2016 to February 2017). Patients were followed for 12 weeks after enrollment (Fig. 1) and the FACT/GOG-Ntx questionnaire scores were collected at baseline (within 2 weeks of cycle 1 of chemotherapy), week 6, and week 12. To accommodate standard scheduling practices for clinic visits and outpatient infusion clinic appointments, a 2-week margin was used for follow-up assessments. Therefore, the week 6 appointment could occur from weeks 4 to 8, and the week 12 appointment could occur from weeks 10 to 14.
Data collection
Demographics, FACT/GOG-Ntx score, grade of neuropathy, previous chemotherapy within 1 year prior to study enrollment, medications for peripheral neuropathy, depression, over-the-counter supplements or vitamins, and medical comorbidities were obtained from electronic medical record and patient-completed questionnaire during enrollment at baseline. The FACT/GOG-Ntx questionnaire and medication history were completed by the patient during follow-up assessment at week 6 and week 12.
Measure of CIPN and QOL
The FACT/GOG-Ntx version 4 questionnaire which assesses QOL and neurotoxicity is a validated, subjective, patient-based tool for CIPN assessment with good psychometric properties [26, 32]. The neurotoxicity subscale (NtxS) is an 11-item questionnaire where patients report sensory, motor, and auditory symptoms on a Likert scale of 0 (not at all) to 4 (very much); possible total scores range from 0 to 44, with a higher score reflecting less neurotoxicity [26, 32]. Literature does not clearly define clinically meaningful change for the NtxS [33]. A previous study determined that patients with known CIPN had FACT/GOG-Ntx scores that were 10% lower than patients who were chemotherapy-naïve [26]. The QOL assessment consists of 27 questions subdivided into 4 categories: physical well-being, social/family well-being, emotional well-being, and functional well-being [26, 32]. Patients report on a Likert scale of 0 (not at all) to 4 (very much) with a possible total scores range of 0–108; higher score indicates a higher quality of life [26, 32]. The FACT/GOG-Ntx score combines the NtxS and QOL scores with a possible score range from 0 to 152 [26, 32]. For this study, clinically significant development of CIPN was defined as a decrease in the NtxS by > 10% between 2 time points (weeks 0 to 6 and weeks 0 to 12).
Prespecified subgroup analysis
This non-interventional study also conducted a prespecified subgroup analysis. As part of routine care, one hematologist prescribed a combination of OTC supplements (alpha lipoic acid, thiamine, and pyridoxine) with written instruction on dosing and frequency in an attempt to prevent development and severity of CIPN; this was not assigned as part of the study protocol. This combination of OTC supplements included alpha lipoic acid 600 mg once daily, thiamine 100 mg once daily, and pyridoxine 50 mg once daily. To provide preliminary data on combination OTC supplements, patients were stratified into two groups: patients who did and did not receive this combination of OTC supplements. This small group of patients (n = 6) formed a natural subgroup.
Statistical analyses
The primary analysis was the change in NtxS from baseline to week 6 and from baseline to week 12 was performed using a paired t test. Secondary analyses of change in QOL and FACT/GOG-Ntx scores from baseline to week 6 and from baseline to week 12 were performed using a paired t test. Subgroup analyses were performed among strata of OTC supplement status (receiving versus not receiving), diagnosis, and chemotherapy type. A two-sided alpha of 0.05 was used to test for statistical significance. Analyses were performed using Stata version 15.1 (StataCorp LP, College Station, TX, USA).
Results
Accrual and eligibility
Of 92 patients screened, 39 (42%) patients were eligible for the study (Fig. 1). Six patients were not approached for informed consent due to the following reasons: medical instability (n = 2), social situation (n = 2), and investigator unavailability (n = 2). Of 33 patients approached, 28 (85%) provided informed consent and were enrolled in this study. Between baseline and week 6, three patients were lost to follow-up (two patients changed healthcare providers per insurance preference and one patient expired). Between week 6 and week 12, one patient declined the survey. Over the course of this study, 28 patients completed assessment at baseline, 25 patients (89%) completed the week 6 assessment, and 24 patients (86%) completed the week 12 assessment.
Feasibility and time to completion of informed consent and questionnaires
Twenty-one patients completed initial assessment during an outpatient clinic visit, and 7 patients completed initial assessment during an inpatient admission. Investigators documented time-in-motion data for 22 (79%) of 28 of informed consent discussions, 20 (71%) of 28 eligible baseline questionnaires, 20 (80%) of 25 eligible week 6 questionnaires, and 23 (96%) of 24 eligible week 12 questionnaires. The mean (standard deviation) duration of informed consent was 15 (4) minutes per patient. The mean (SD) duration of completing the FACT/GOG-Ntx questionnaire was 22 (4) minutes at baseline, 16 (3) minutes at week 6, and 15 (4) minutes at week 12.
Patient characteristics
Study patients had a mean (SD) age of 60 (17) years, and were predominately male (64%) and white (64%) (Table 1). Sixty-eight percent had lymphoma and 32% had multiple myeloma. The majority (70%) of patients had not previously received chemotherapy.
Primary endpoint: changes in NtxS
Among 28 patients in this study, NtxS changed from baseline by − 2.7 points (95% CI − 5.5 to 0.1; p = 0.061) at week 6 (n = 25) and by − 6.0 points (95% CI − 8.8 to − 3.2; p < 0.001) at week 12 (n = 24) (Table 2). A clinically significant development of CIPN (decrease of > 10% from baseline) in neurotoxicity score from baseline was detected in 9 (36%; 95% CI 18 to 57%) of 25 eligible patients at week 6 and in 16 (67%; 95% CI 45 to 84%) of 24 eligible patients at week 12. The rate of decrease of NtxS was 3 points for each 6-week period, and this was consistent for both time periods from weeks 0 to 6 and from weeks 6 to 12. Long-term follow-up of NtxS beyond week 12 may be warranted.
Secondary endpoints: changes in QOL and FACT/GOG-Ntx scores
Among 28 patients in this study, QOL scores changed from baseline by − 3.9 points (95% CI − 8.7 to 0.8; p = 0.101) at week 6 (n = 25) and by − 12.1 points (95% CI − 18.6 to − 5.7; p < 0.001) at week 12 (n = 24) (Table 3). The QOL scores decreased more rapidly in the time period from weeks 6 to 12 than what was observed from weeks 0 to 6. Long-term follow-up of QOLs scores beyond week 12 may be warranted. The mean change in FACT/GOG-Ntx score (combination of NtxS and QOL) from baseline to week 6 (n = 25) was − 6.6 points (95% CI − 13.2 to − 0.1; p = 0.048) and from baseline to week 12 (n = 24) was − 18.1 points (95% CI − 25.2 to − 11.0; p < 0.001) (Table 3).
Subgroup analysis on the combination of OTC supplements
Of 28 included patients, 6 (21%) received the combination of OTC supplements during the entire duration of the study (baseline to week 12) and 22 (79%) did not received the combination of OTC supplements (Table 1). All the six patients taking combination OTC supplement were also taking L-carnitine supplements. None of the patients started a new OTC supplement during the study. At week 6 and week 12 visits, all 6 patients in this subgroup reported that they were still actively taking these prescribed OTC supplements.
Changes in NtxS based on combination OTC supplement status
The combination of OTC supplements was prescribed for 21% (6 of 28) of study patients, who were older, more likely to have multiple myeloma, more likely to receive bortezomib, and less likely to receive vincristine than patients who did not take the combination of OTC supplements. Among 22 patients who did not receive the combination of OTC supplements, NtxS changed from baseline by − 2.9 points (95% CI − 5.5 to − 0.4; p = 0.025) at week 6 (n = 19) and by − 6.4 points (95% CI − 10.2 to − 2.7; p = 0.002) at week 12 (n = 18) (Table 2) (Fig. 2). Among 6 patients who received the combination of OTC supplements, NtxS changed from baseline by − 1.8 points (95% CI − 13.5 to 9.8; p = 0.702) at week 6 (n = 6) and by − 4.5 points (95% CI − 7.7 to − 1.3; p = 0.015) at week 12 (n = 6) (Fig. 2). A clinically significant development of CIPN (decrease of > 10% from baseline) in neurotoxicity score from baseline were detected in 1 (17%; 95% CI 0.4 to 64%) of 6 patients who took OTC supplements at week 6, 8 (42%; 95% CI 20 to 67%) of 19 patients who did not take OTC supplements at week 6, 3 (50%; 95% CI 12 to 88%) of 6 patients who took OTC supplements at week 12, and 13 (72%; 95% CI 47 to 90%) of 18 patients who did not take OTC supplements at week 12.
Changes in NtxS and QOL scores over time. NtxS, neurotoxicity subscale; QOL, quality of life; OTC, over the counter. Panel A: Black dots represent patients who did not receive combination OTC supplements and gray dots represent patients who received combination OTC supplements. Panel B: Black squares represent patients who did not receive combination OTC supplements and gray squares represent patients who received combination OTC supplements
QOL score based on combination OTC supplement status
Among 22 patients who did not receive the combination of OTC supplements, QOL changed from baseline by − 2.9 (95% CI − 7.6 to 1.8; p = 0.215) at week 6 (n = 19) and by − 11.9 points (95% CI − 19.6 to − 4.1; p = 0.005) at week 12 (n = 18) (Table 3) (Fig. 2). Among 6 patients who received the combination of OTC supplements, QOL changed from baseline by − 7.4 (95% CI − 25.1 to 10.3; p = 0.333) at week 6 (n = 6) and by − 12.9 points (95% CI − 29.6 to 3.7; p = 0.101) at week 12 (n = 6) (Table 3) (Fig. 2).
Subgroup analyses by cancer type and chemotherapeutic agent
Vincristine was the most commonly used chemotherapy agent in this study cohort, and all 17 patients treated with vincristine had lymphoma (Table 4). Among these patients, NtxS changed from baseline by − 2.1 points (95% CI − 5.5 to 1.2; p = 0.194) at week 6 (n = 15) and by − 5.9 points (95% CI − 10.5 to − 1.2; p = 0.018) at week 12 (n = 14) compared with baseline. The QOL scores changed from baseline by − 1.7 points (95% CI − 7.3 to 3.9; p = 0.523) at week 6 (n = 15), and by − 9.2 points (95% CI − 19.0 to 0.6; p = 0.065) at week 12 (n = 14) from the baseline.
Bortezomib was the second most commonly used chemotherapy agent in this study cohort, and 7 of 8 patients who received bortezomib had multiple myeloma. Of these 7 patients, NtxS changed from baseline by − 5.6 points (95% CI − 13.6 to 2.4; p = 0.140) at week 6 (n = 7), and by − 7.7 points (95% CI − 11.6 to − 3.8; p = 0.003) at week 12 (n = 7) compared with baseline. The QOL scores changed from baseline by − 9.4 points (95% CI − 23.8 to 5.0; p = 0.162) at week 6 (n = 7), and by − 18.9 points (95% CI − 31.4 to − 6.5; p = 0.010) at week 12 (n = 7) compared with baseline.
Documentation of physician assessment of peripheral neuropathy in medical record
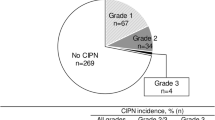
Physician notes in the electronic medical record for documentation of assessment of development of CIPN were reviewed for all study patients at weeks 6 (n = 25) and week 12 (n = 24). At week 6, 7 (28%) patients had documentation of CIPN (grade 1 for 2 patients; grade not specified for 5 patients). At week 12, 10 (42%) patients had documentation of CIPN (grade 1 for 1 patient; grade 2 for 2 patients, grade not specified for 7 patients). Compared with the reference standard of clinically significant development of CIPN (NtxS decreased by > 10% from baseline), physician documentation of any grade of CIPN had a sensitivity of 44% (95% CI 14 to 79%) and specificity of 81% (95% CI 54 to 96%) at week 6 and a sensitivity of 50% (95 CI 25 to 75%) and specificity of 75% (95% CI 35 to 97%) at week 12.
Discussion
This pilot, feasibility study demonstrated that patients can be successfully enrolled in a study characterizing the development of CIPN in both the inpatient and outpatient oncology settings. The proportion of eligible patients who provided consent was 85%, and the proportion of enrolled patients who completed all CIPN assessments over 12 weeks was 86%. The data from investigator-documented time-in-motion records of duration of informed consent and questionnaire completion provides information regarding the time commitment for planning future studies. The results of this study were interpreted as favorable by the study team, and several clinicians on the study team have requested that the health-system’s governance committees of our institution consider enhancing the electronic medical record to include the FACT/GOG-Ntx for routine screening. The average completion time for the FACT/GOG-Ntx questionnaire was 15–22 min. One perceived barrier that needs to be addressed prior to implementing FACT/GOG-Ntx screening during routine care would be efficient integration of screening into the clinic workflow so that nurses are not overburdened and that patients’ time in the clinic is not unnecessarily prolonged. The option to integrate the 11-item questionnaire neurotoxicity subscale versus the complete FACT/GOG-Ntx with 38 questions will be considered to reduce patient time needed to complete the assessment.
This study highlights the importance of prospectively screening for CIPN using a validated screening tool (FACT/GOG-Ntx), as physician progress notes did not capture all clinically meaningful cases of CIPN. Literature shows that despite being a potentially serious adverse effect of chemotherapy, it is underreported [4]. Objective findings and patient assessments have shown superiority in detecting neuropathy symptoms in comparison to physician-rated results [34]. Patient-based assessment tools, like the FACT/GOG-Ntx survey, assist in detecting the presence and severity of neurotoxicity in these patients [4]. This study further supports the simplicity and utility of the FACT/GOG-Ntx questionnaire in characterizing the development of CIPN over time [26]. This study defined a clinically significant decrease in NtxS to be a decrease of > 10% from baseline, as a 10% reduction in overall FACT/GOG-Ntx may reflect CIPN [26]; however, this particular threshold has not been validated in the NtxS portion of the FACT/GOG-Ntx. The trend of NtxS from baseline to week 6 and week 12 is consistent with previous literature about development of CIPN in a time-dependent and cumulative dose-dependent manner [7, 12, 26, 35].
Estimates of NtxS and QOL changes over time must be interpreted with caution among the subgroup analysis of patients taking a combination of OTC supplement, as the sample size in this group was small (n = 6), which did not allow for statistical adjustment of potential confounders. Patients in this subgroup were more likely to receive bortezomib and have multiple myeloma, and the sample was not large enough to allow for statistical adjust of this imbalance. However, this data can be used to estimate statistical variations in scores over time and between patients, which can be used to inform the sample size calculation of a future clinical trial that is designed to evaluate an intervention to prevent CIPN. Clinically significant CIPN (NtxS decrease by > 10% from baseline) was observed at week 12 among 50% (3 out of 6) of patients who received the combination of OTC supplements and among 72% (13 out of 18) of patients who did not. Evaluation of outcomes using this subgroup analysis could be at risk for selection bias.
Limitation
This was a single-center, pilot, feasibility study that was not designed to compare NtxS or QOL trends between subgroups of patients based on disease or medication exposure. Adherence to OTC supplements was not measured. Although assessing for CIPN at week 6 and week 12 appears to clinically meaningful, extending the follow-up period could provide additional information on development of CIPN that present later in the course of therapy. Even though the FACT/GOG-Ntx questionnaire is a validated tool for assessing CIPN, it remains a subjective approach, due to patient bias and scoring of the questionnaire. Since this study was developed to test feasibility, the study had a small sample size that limited precision and power of the analyses.
Conclusion
Clinically significant CIPN was detected in 67% within 12 weeks of starting neurotoxic chemotherapy for adults with newly diagnosed with lymphoma or multiple myeloma. Feasibility metrics for enrollment, consent, CIPN assessment, and 12-week follow-up demonstrated that it would be feasible to plan future studies. Physician documentation of CIPN in the medical record underreported the incidence of CIPN detected using prospective screening with the FACT/GOG-Ntx validated instrument, which underscores the importance of implementing prospective screening with a validated tool for clinical practice and future studies.
References
Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL (2016) National Cancer Institute-supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer 24(3):1439–1447. https://doi.org/10.1007/s00520-015-3063-4
Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155(12):2461–2470. https://doi.org/10.1016/j.pain.2014.09.020
Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F (2006) Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 33(1):15–49. https://doi.org/10.1053/j.seminoncol.2005.12.010
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63(6):419–437. https://doi.org/10.3322/caac.21204
Brewer JR, Morrison G, Dolan ME, Fleming GF (2016) Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol 140(1):176–183. https://doi.org/10.1016/j.ygyno.2015.11.011
Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB (2012) Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract 2012:1–10
Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, Zanna C (2007) The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst 12(3):210–215. https://doi.org/10.1111/j.1529-8027.2007.00141.x
Velasco R, Bruna J (2010) Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia (Barcelona, Spain) 25(2):116–131
Griffith KA, Couture DJ, Zhu S, Pandya N, Johantgen ME, Cavaletti G, Davenport JM, Tanguay LJ, Choflet A, Milliron T, Glass E, Gambill N, Renn CL, Dorsey SG (2014) Evaluation of chemotherapy-induced peripheral neuropathy using current perception threshold and clinical evaluations. Support Care Cancer 22(5):1161–1169. https://doi.org/10.1007/s00520-013-2068-0
Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159(2):327–333. https://doi.org/10.1007/s10549-016-3939-0
Tofthagen C, Overcash J, Kip K (2012) Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer 20(3):583–589. https://doi.org/10.1007/s00520-011-1127-7
Zanville NR, Nudelman KN, Smith DJ, Von AD, McDonald BC, Champion VL, Saykin AJ (2016) Evaluating the impact of chemotherapy-induced peripheral neuropathy symptoms (CIPN-sx) on perceived ability to work in breast cancer survivors during the first year post-treatment. Support Care Cancer 24(11):4779–4789. https://doi.org/10.1007/s00520-016-3329-5
Kazandjian D (2016) Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol 43(6):676–681. https://doi.org/10.1053/j.seminoncol.2016.11.004
Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364(11):1046–1060. https://doi.org/10.1056/NEJMra1011442
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR (2016) 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 66:443–459. https://doi.org/10.3322/caac.21357
American Society of Clinical Oncology (2019) Multiple myeloma: statistics. https://www.cancer.net/cancer-types/multiple-myeloma/statistics. Accessed 29 Mar 2019
Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine DL, Schenkein DP, Anderson KC (2004) A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127(2):165–172. https://doi.org/10.1111/j.1365-2141.2004.05188.x
Pal PK (1999) Clinical and electrophysiological studies in vincristine induced neuropathy. Electromyogr Clin Neurophysiol 39(6):323–330
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348(26):2609–2617. https://doi.org/10.1056/NEJMoa030288
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA (2006) Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 24(19):3113–3120. https://doi.org/10.1200/JCO.2005.04.7779
Ropper AH, Gorson KC (1998) Neuropathies associated with paraproteinemia. N Engl J Med 338(22):1601–1607. https://doi.org/10.1056/NEJM199805283382207
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B (1999) Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341(21):1565–1571. https://doi.org/10.1056/NEJM199911183412102
Trivedi MS, Hershman DL, Crew KD (2015) Management of chemotherapy-induced peripheral neuropathy. Am J Hematol-Oncol 11(1):4–10
Curcio KR (2016) Instruments for assessing chemotherapy-induced peripheral neuropathy: a review of the literature. Clin J Oncol Nurs 20(2):144–151. https://doi.org/10.1188/16.cjon.20-01ap
Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D (2003) Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13(6):741–748
Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P, Cortinovis D, Bidoli P (2010) Chemotherapy-induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer (Oxford, England : 1990) 46(3):479–494. https://doi.org/10.1016/j.ejca.2009.12.008
Abbas ZG, Swai AB (1997) Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East Afr Med J 74(12):803–808
Cakici N, Fakkel TM, van Neck JW, Verhagen AP, Coert JH (2016) Systematic review of treatments for diabetic peripheral neuropathy. Diabet Med 33(11):1466–1476. https://doi.org/10.1111/dme.13083
Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schutte K, Dyck PJ (2011) Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 34(9):2054–2060. https://doi.org/10.2337/dc11-0503
Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32(18):1941–1967. https://doi.org/10.1200/jco.2013.54.0914
Huang HQ, Brady MF, Cella D, Fleming G (2007) Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer 17(2):387–393. https://doi.org/10.1111/j.1525-1438.2007.00794.x
Spigel DR, Patel JD, Reynolds CH, Garon EB, Hermann RC, Govindan R, Olsen MR, Winfree KB, Chen J, Liu J, Guba SC, Socinski MA, Bonomi P (2015) Quality of life analyses from the randomized, open-label, phase III PointBreak study of pemetrexed-carboplatin-bevacizumab followed by maintenance pemetrexed-bevacizumab versus paclitaxel-carboplatin-bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Thorac Oncol 10(2):353–359. https://doi.org/10.1097/JTO.0000000000000277
Stubblefield MD, Burstein HJ, Burton AW, Custodio CM, Deng GE, Ho M, Junck L, Morris GS, Paice JA, Tummala S, Von Roenn JH (2009) NCCN task force report: management of neuropathy in cancer. J Natl Compr Cancer Netw 7(Suppl 5):S1–S26 quiz S27-28
Driessen CM, de Kleine-Bolt KM, Vingerhoets AJ, Mols F, Vreugdenhil G (2012) Assessing the impact of chemotherapy-induced peripheral neurotoxicity on the quality of life of cancer patients: the introduction of a new measure. Support Care Cancer 20(4):877–881. https://doi.org/10.1007/s00520-011-1336-0
Acknowledgments
The authors would like to thank Drs. Lawrence Rice, Swaminathan Iyer, Ibrahim Ibrahim, Sai Ravi Pingali, Kirk Heyne, and Eric Bernicker Hematology/Oncology Physicians at Houston Methodist Hospital for giving the investigators permission to approach their patients for enrollment in this study. The authors would like to thank Marcus Scott, infusion clinic scheduler, and David Raven, RN, at Houston Methodist Hospital, for helping with patient identification.
Meetings
A research poster containing study results were presented at the Vizient® Pharmacy Network meeting in Las Vegas, Nevada, in December 2016. A research poster was presented at the Hematology/Oncology Pharmacy Association Conference in Anaheim, CA, in March 2017. A platform presentation of study results was presented at the Midwest Pharmacy Residency Conference in Omaha, NE, in May 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflicts of interest to disclose related to this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajewole, V.B., Cox, J.E., Swan, J.T. et al. Incidence of chemotherapy-induced peripheral neuropathy within 12 weeks of starting neurotoxic chemotherapy for multiple myeloma or lymphoma: a prospective, single-center, observational study. Support Care Cancer 28, 1901–1912 (2020). https://doi.org/10.1007/s00520-019-05006-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05006-6