Abstract
Purpose
In this study, we aimed to estimate the frequency of deglutition disorders in patients pre- and post-treatment for head and neck cancer (HNC).
Methods
Search strategies were developed for the following databases: LILACS, PubMed, SpeechBITE, LIVIVO, Web of Science, and Scopus. Additionally, the gray literature was searched using Google Scholar, OpenGrey, and ProQuest. Only studies that conducted an evaluation of deglutition before and after cancer treatment and had sufficient quantitative data were included. We conducted a proportion of random effects meta-analysis using R statistical software.
Results
Seventeen studies were included. Aspiration showed a high frequency in the period less than 3 months post-treatment, with 28.6% (total sample = 229). Penetration of fluids above the vocal folds and reduced laryngeal elevation were more frequent in the period less than 6 months post-treatment.
Conclusion
The frequency of deglutition disorders and its complications, such as aspiration, appears to be higher in the immediate to 6-month post-treatment period in patients with HNC. The parameter pharyngeal residue continued to increase through the period analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swallowing assessment and treatment have become common practice in patients with head and neck cancer (HNC). However, there is no consensus as to when assessment of deglutition disorders should begin, which methods to use in the evaluation, when and how to start treatment options, and for how long patients should be followed up [1].
The frequency of deglutition disorders varies with the cancer etiology and/or type of cancer treatment. HNC is the sixth most common cancer worldwide, accounting for 2.8% of all malignancies. Treatments available for HNC can include surgery, radiotherapy, chemotherapy, or a combination of treatments [2]. The choice of treatment modality is dependent of patient variables, primary site, clinical stage, and resectability of the tumor. The adverse effects or toxicities of these treatments may include dysphagia and many other disorders that can have an impact on the swallowing function, such as pain, dry mouth, mucositis, dysgeusia, nausea, and loss of appetite [3,4,5,6,7]. Dysphagia and aspiration are recognized as potentially devastating complications of treatment of HNC.
The frequency of dysphagia in HNC differs when considering different variables such as radiotherapy field size and radicality of surgery. Dysphagia has a higher prevalence (63.6%) early following surgery (< 1 year) than delayed (> 5 years) [8]. Similarly, dysphagia has a prevalence of 45.9% after cancer treatment (surgery and/or radiotherapy) [9].
Two previous systematic reviews [10, 11] investigated the changes in swallowing mechanisms after radiation or drug therapy in HNC. Quantitative analysis was not performed in either review. Further research on this topic is needed, as new and more detailed studies have surfaced over the last 5 years [12,13,14,15,16,17]. There is also a need for quantitative analysis of the results found in the literature. The aim of this systematic review was therefore to answer the research question: What is the frequency of deglutition disorders pre- and post-treatment among patients who undergo therapy for HNC?
Methods
This systematic review (SR) was reported following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [18].
Protocol and registration
The SR protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42017067837 [19].
Eligibility criteria
Inclusion criteria
We included studies that measured the frequency of deglutition disorders pre- and post-treatment in patients with head and neck neoplasms that received any type of therapy for cancer (surgery, chemotherapy, radiotherapy, or a combination of therapies). We included only those studies with participants over 18 years old and those who had undergone imaging exams (i.e., videofluoroscopic swallowing study (VFSS)) as diagnostic criteria for the assessment of deglutition disorders and complications. No language or period restriction was applied.
Exclusion criteria
Studies were excluded for the following reasons: (1) patients without cancer or non-malignant tumors; (2) studies that did not use imaging exams such as VFSS or fiber-optic endoscopic evaluation of swallowing (FEES) as diagnostic criteria for deglutition disorders before and after cancer treatment; (3) patients who did not undergo any type of cancer treatment or therapy; (4) patients already receiving treatment for deglutition disorders; (5) studies reporting insufficient quantitative data to perform analysis of deglutition disorders; (6) reviews, letters, conference abstracts, opinions, case reports, and cross-sectional studies; (7) full text unavailable; and (8) duplicate data from another study.
Information sources
For the literature search, an individual strategy was developed for each of the following databases: LILACS, PubMed, SpeechBITE, LIVIVO, Web of Science, and Scopus. An additional search of the gray literature was performed using Google Scholar, OpenGrey, and ProQuest. The search date was 23 March 2017 for all databases and gray literature. An updated search was performed on 19 June 2018. The search strategies used are described in Appendix 1. The references cited in the included articles were checked for any additional studies that could be included in the analysis, as recommended by Greenhalgh and Peacock [20]. Studies were collected using reference manager software (EndNote™ Online, Thomson Reuters, Philadelphia, PA, USA). Duplicate studies were identified with the software and posteriorly, any additional duplicates not identified by EndNote were found with the help of Rayyan QCRI, a free web, and mobile app for systematic reviews (Qatar Computing Research Institute, Doha, Qatar) [21].
Study selection
The process of reference selection was divided into two phases. Phase 1 was performed by two reviewers (I.P.T. and L.Q.P.), who independently screened the title and abstract of the collected studies. This blind process was ensured and registered as it was carried out using the Rayyan QCRI platform. Studies that did not fit the inclusion criteria were excluded. In phase 2, the same reviewers (I.P.T. and L.Q.P.) applied the eligibility criteria to the full text of the studies selected in phase 1. When necessary, a third reviewer (K.F.L.) was consulted to reach a consensus in cases of disagreement between the first two reviewers.
Data collection process
The first reviewer (I.P.T.) collected the required information from the selected studies. The second reviewer (L.Q.P.) cross-checked all the collected information for accuracy. The data collected consisted of: study characteristics (authors, year of publication, country, journal of publication, type of study); population characteristics (sample size, age, type of cancer, cancer stage); exposure characteristics (type of cancer treatment, deglutition assessment); and outcome characteristics (occurrence of deglutition disorders pre-treatment and post-treatment).
Risk of bias in individual studies
The risk of bias in individual studies was assessed using the JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data [22]. The first and second reviewers (I.P.T. and L.Q.P.) performed this assessment independently. Any disagreements were resolved in discussions among the three first reviewers (I.P.T., L.Q.P., and K.F.L.).
Subgroup analysis
The analysis was planned in subgroups, dividing the deglutition parameters according to the period of assessment with imaging exams (VFSS or FEES) as follows: pre-treatment, less than 6 months post-treatment (from less than 3, 4, and 6 months), at 6 months post-treatment, and post-treatment more than 6 months (from 6 to 12 months).
Summary measures
The data collected for deglutition disorders or complications (aspiration and liquid penetration, among others) in adult patients with HNC who underwent cancer treatment were expressed using mean percentage and 95% confidence interval (CI).
Synthesis of results
A meta-analysis was planned, including those studies that presented sufficient data to determine the frequency of deglutition disorders or complications pre- and post-treatment. These data were analyzed using random effects meta-analysis [23]. Calculations were performed using R Statistical Software version 3.4.2 (The R Foundation, Vienne, Austria). The packages utilized were “metaphor” and “meta,” including arcsine transformation to calculate the overall proportion; in addition, the Clopper–Pearson interval was used to calculate 95% CIs. Heterogeneity was calculated using an inconsistency index (I2), and a value greater than 50% was considered an indicator of substantial heterogeneity within studies [24]. The significance level was set at 5%.
Results
Study selection
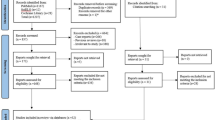
In phase 1 of this systematic review, 1368 records were screened from the six main databases, after removing duplicates. An additional 302 records from the gray literature were included. After screening all titles and abstracts, 171 studies were selected for phase 2, which consisted of full-text screening. In this phase, a total of 155 studies were excluded (Appendix 2). No additional articles were selected from the reference list review. In the updated search, 99 studies were screened but only 1 met the inclusion criteria. Finally, 17 studies were included in the qualitative and quantitative analysis. The study selection process is depicted in Fig. 1.
Study characteristics
The 17 included articles [12,13,14,15,16,17, 25,26,27,28,29,30,31,32,33,34,35] were published in 10 journals, with a quarter of these [13, 29, 32, 33] published in the journal Head & Neck, four [14, 25, 26, 35] published in Dysphagia, and two [28, 30] studies published in The Laryngoscope. The remaining seven studies were published in different oncology, medical, or speech-language pathology journals. The total sample varied from 11 [28] to 133 [16] patients. Sixteen [12, 14,15,16,17, 25,26,27,28,29,30,31,32,33,34,35] of the included studies used a VFSS as a diagnostic tool for deglutition disorders, and one [13] study used a FEES. Nearly half [14, 27,28,29, 31,32,33] of the studies were from the USA, two [12, 15] were from Turkey, and there was one each from Australia [26], Canada [34], China [30], India [25], Korea [16], The Netherlands [17], the UK [13], and Greece [35]. Because of the nature of the research question of this systematic review, all of the included studies used a convenience sample. A summary of the characteristics of the 17 included studies can be found in Table 1, and a summary of the quantitative data is given in Table 2.
Risk of bias within studies
Ten [13, 15,16,17, 25, 29, 32,33,34,35] of the included studies were classified as having a low risk of bias. Six [14, 26,27,28, 30, 31] other studies were considered to have a moderate risk of bias, with answers of “yes” to five or six of the ten questions in the assessment tool. Only one [12] study was assessed as having a high risk of bias. All studies produced negative responses to the question “Were study participants recruited in an appropriate way?” because all samples were convenience samples. Nearly half [12, 25,26,27, 30, 32] of the assessed studies yielded “no” responses to the question “Are all important confounding factors/subgroups/differences identified and accounted for?,” which points out a lack of information in the description of the sample and/or selection criteria. Details of study classification can be found in Appendix 3.
Results of included studies
Overall data distribution
All samples in the included studies had a higher proportion (at least two thirds) of male participants than female ones. The most frequent types of cancer reported in the included studies were cancer of the oropharynx, tongue, larynx, hypopharynx, nasopharynx, tonsil, unknown sites, and others. Most studies that presented data concerning cancer stages grouped participants according to stages III–IV. Eleven [12, 13, 25,26,27, 29,30,31,32,33, 35] of the included studies reported concurrent chemoradiotherapy as the main treatment modality for cancer; the remaining five [14,15,16, 28, 34] studies reported the modalities surgery and conventional radiotherapy and/or chemotherapy. Only one [17] of the included studies presented data for intensity-modulated radiation therapy (IMRT) combined with chemotherapy.
Subgroup analysis
The analysis of deglutition parameters was divided into subgroups. These main groups were selected according to the period in which swallowing assessment with imaging exams (VFSS or FEES) was performed. In all the included studies, one of these exams was performed at baseline and at least once after treatment for HNC. The meta-analysis was then classified according to swallowing parameters, as assessed in imaging exams during the different time periods: pre-treatment, less than 6 months post-treatment (from less than 3, 4, and 6 months), at 6 months post-treatment, and more than 6 months post-treatment (from 6 to 12 months).
Pre-treatment
All 17 included studies performed swallowing assessment using imaging exams at baseline. The types of parameters concerning deglutition were reported in different ways across studies. The most frequently reported parameter in 14 of the included studies [12, 13, 15,16,17, 25, 27,28,29,30,31,32, 34, 35] was aspiration, a complication of deglutition disorders. The results of meta-analysis at pre-treatment showed a frequency of aspiration of 8.4% (95% CI 4.3–13.7; p < 0.01; n = 572; I2 = 74%) (Fig. 2a).
a–e Meta-analysis graphs and data for aspiration in different time periods. f–h Meta-analysis graphs and data for penetration in different time periods. i–m Meta-analysis graphs and data for reduced laryngeal elevation in different time periods. n–q Meta-analysis graphs and data for pharyngeal residue in different time periods
In five [12, 14, 27, 29, 30] of the included studies, it was possible to analyze the total occurrence of penetration of food, liquids, or saliva above the vocal folds in the pre-treatment period. According to results of the analysis, the frequency of penetration was of 10.5% (95% CI 3.3–21.0; p = 0.07; n = 99; I2 = 54%) (Fig. 2f).
Two other important parameters related to deglutition disorders are the reduction in elevation of the larynx and pharyngeal residue. Meta-analysis was performed for reduced laryngeal elevation in eight studies [12, 16, 20, 26, 28, 29, 33, 35] at baseline, with a frequency for this parameter of 16.0% (95% CI 7.4–27.0; p < 0.01; n = 301; I2 = 80%) (Fig. 2i). For pharyngeal residue, a total of seven studies [12, 16, 25, 27, 29, 30, 35] were included in the analysis, with pre-treatment frequency of 12.7% (95% CI 0.9–34.9; p < 0.01; n = 362; I2 = 96%) (Fig. 2n).
Up to 6 months post-treatment
All parameters analyzed in the pre-treatment period were also assessed for less than and at 6 months post-treatment. In the meta-analysis for aspiration at less than 3 months post-treatment, the frequency was 28.6% (95% CI 21.7–36.1; p = 0.20; n = 229; I2 = 30%) (Fig. 2b). Similarly, frequency in the period more than 3 months post-treatment reached 28.2% (95% CI 19.3–38.0; p < 0.01; n = 361; I2 = 72%) (Fig. 2c). At 6 months post-treatment, this frequency declined to 17.6% (95% CI 19.3–38.0; p < 0.01; n = 125; I2 = 88%) (Fig. 2d). The frequency of penetration for the period less than 6 months post-treatment was 41.3% (95% CI 19.7–64.9; p < 0.01; n = 79; I2 = 78%) (Fig. 2g). The parameter reduced laryngeal elevation at less than 4 months post-treatment had a frequency of 46.2% (95% CI 17.0–76.9; p < 0.01; n = 105; I2 = 90%) (Fig. 2j). This proportion dropped to 27.3% (95% CI 12.8–44.8; p < 0.01; n = 219; I2 = 85%) at less than 6 months post-treatment (Fig. 2k) and increased slightly to 29.7% (95% CI 14.5–47.6; p = 0.08; n = 81; I2 = 60%) at 6 months (Fig. 2l) post-treatment. The pharyngeal residue parameter showed increasing frequencies over time; at less than 6 months this was 47.1% (95% CI 27.9–66.8; p < 0.01; n = 331; I2 = 92%) (Fig. 2o) and at 6 months the frequency was increased to 61.8% (95% CI 30.2–88.7; p < 0.01; n = 145; I2 = 93%) (Fig. 2p).
More than 6 months post-treatment
There were few studies included in meta-analysis of the four parameters for the period more than 6 months post-treatment. Many included studies did not follow the patient for more than 6 months after receiving treatment for cancer. In the analysis for aspiration, a total of six [17, 25, 27, 30, 34, 35] studies had data collected, only half as many as in the baseline analysis. The frequency of aspiration from 6 to 12 months post-treatment was 16.2% (95% CI 8.1–26.3; p = 0.03; n = 158; I2 = 60%) (Fig. 2e). Similarly, for the parameter penetration, only three [12, 27, 30] studies were included in the meta-analysis and showed a frequency of 33.6% (95% CI 5.7–70.4; p < 0.01; n = 66; I2 = 90%) (Fig. 2h). For the reduction of laryngeal elevation, the frequency was calculated for three studies [27, 33, 35], reaching 23.4% (95% CI 11.0–38.7; p = 0.04; n = 113; I2 = 68%) (Fig. 2m). The last parameter calculated was pharyngeal residue, with four included studies [25, 27, 30, 35] in the analysis and a frequency of 73.8% (95% CI 45.2–94.3; p < 0.01; n = 122; I2 = 91%), the highest frequency found across periods and parameters (Fig. 2q).
Synthesis of results
A proportion meta-analysis was conducted among the 17 included studies. Data of the analysis performed are shown in Fig. 2a–q. The heterogeneity between studies varied from 30 to 96%; therefore, a random effects model was chosen [23]. Most higher frequencies of deglutition complications were found in the two periods less than and at 6 months post-treatment. The only exception to this was pharyngeal residue, which presented the highest frequency in the period more than 6 months post-treatment. A higher frequency of deglutition disorders was observed across studies among patients who underwent combination therapy (surgery, radiotherapy, chemotherapy), as compared with patients who only underwent concurrent chemoradiation.
Level of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for evaluating the quality of evidence was adapted for the analysis of observational studies and applied to subgroups, according to the meta-analysis performed for deglutition parameters before and after cancer treatment. All the outcomes across subgroups were graded as low quality, reflecting the heterogeneity of the meta-analysis, evidenced by the different types of HNC topographies and cancer treatments. A GRADE table of findings is shown in Appendix 4.
Discussion
With the increasing use of aggressive combined modality therapy and radiation techniques for the treatment of locally advanced head and neck cancer, the acute and late effects of treatment have become an area of interest and investigation. Thus, the objective of this SR was to assess the frequency of deglutition disorders in the population of patients with HNC. We investigated parameters of deglutition disorders and complications that were diagnosed using imaging exams for swallowing function. The results indicated a higher frequency of deglutition complications in the period immediately following cancer treatment, from its completion up to 6 months. This only differed for the parameter pharyngeal residue, which increased over time (from 6 to 12 months). Complications of deglutition alterations could include a higher need of long-term feeding tube and higher risk of aspiration pneumonia with its impact on patient’s quality of life [36].
Currently, in the hierarchy of evidence, meta-analysis is located at the top of the pyramid. This pooled analysis from various individual studies is able to perform a more precise estimate of the outcome researched [37]. The goal of this review was to minimize bias through the selection criteria and performed a meta-analysis. This was achieved; however, there is still considerable bias in the individual studies, especially in the characterization of the samples, as in the separation of deglutition disorders by the cancer site, stage, and treatment.
Toxicity from cancer treatment is classified as acute or late, based on its temporal relationship to treatment. Acute toxicity develops during or shortly after the completion of treatment and is usually temporary. Late toxicity presents months to years after the completion of treatment and is often permanent [38]. The most common long-term complication of RT and chemoradiotherapy for HNC is xerostomia [39]. Late complications may include lymphedema, carotid artery injury, trismus, thyroid disease, and dysphagia, among others [39].
Some studies have reported the prevalence of swallowing disorders, such as dysphagia, to be 50.6% [8] in patients with HNC. Rinkel et al. [40] assessed a sample of 50 patients and found that 30 had oropharyngeal cancer and 79.0% presented swallowing problems after receiving chemoradiation. In the USA, a population-based study found that over 9 million Americans reported swallowing problems in 2012 and that the third most common cause was HNC (4.9%) [41].
Our data were mostly in line with the findings of previous SRs, the first one was developed in 2006 [10] and the later in 2013 [11]. Heterogeneity of the methods to quantify deglutition disorders in the included studies was a common factor found in all three SRs. However, we tried to integrate criteria that helped to minimize this assessment’s heterogeneity and perform a meta-analysis. One of the differences when comparing this review to the previous ones is in the criteria that all the studies included had to present baseline and post-treatment data assessed by an image exam, such as VFSS or FEES.
A total of 81 studies were excluded in the selection process of this review because of the lack of VFSS or FEES assessment before and after cancer treatment (Appendix 2). This data evidences the discrepancies that exist when performing research over swallowing complications. The use of image exams can contribute to better diagnose dysphagia, its severity, and which consistencies are safer for oral ingestion [42].
There was also a deficiency in the use of a common tool to quantify the alterations perceived during the image exams. Only two [25, 26] of the included studies used Penetration Aspiration Scale (PAS) as a mean to quantify levels of penetration/aspiration through an 8-point score (1–2 normal; 3–6 penetration; 7–8 aspiration).
The findings of this SR point out that there is a higher frequency of swallowing complications in patients who undergo surgery, radiation, and/or chemotherapy combined. Furthermore, parameters such as pharyngeal residue are more in evidence in our pooled analysis. Residue in the vallecula and/or the pyriform sinus are linked to the occurrence of penetration/aspiration [43]. A 2016 systematic review [44] that assessed the various pharyngeal residue scales available in the literature highlighted that the severity of pharyngeal residue may indicate the risk for aspiration, in mild residue as low risk and severe residue as high risk. The results found in our study indicates pharyngeal residue as one of the parameters quantified in the meta-analysis that increased over the 12 months period. This result may indicate that pharyngeal mobility, strength, and/or sensitivity could still be more impaired after 12 months post-treatment for the cancer. Hiss and Postma [42] indicate that post-swallow aspiration can occur due to the residue accumulated in the pharynx, due to its incoordination and/or decreased contraction.
Long-term follow-up data is necessary to better understand and assess swallow in head and neck cancer patients, especially in the years after treatment. Fortunately, even with the increase in the pharyngeal residue showed in the pooled data in our review, the aspiration parameter decreased after 6 months post treatment. In a study [45] that performed a long-term follow-up of patients with HNC after cancer treatment, reported late dysphagia for a median 9 years, with a feeding tube present in 21.0% of patients. Another example of long-term follow-up, for over 5 years monitoring survivors of HNC, 53.5% of patients treated with a non-IMRT modality and 22.0% treated with IMRT had dysphagia [46].
Swallowing disorders arising as a result of HNC impairs the quality of life of the patient. Radiotherapy and chemoradiotherapy may affect the oral and pharyngeal phase of swallowing. Strategies such as exercises, maneuvers, IMRT, and cryoprotectors may improve the swallowing function and overall quality of life of the patient. The results of this review indicate that the multidisciplinary team treating HNC must be familiar with the basics of normal swallowing and how swallowing is affected by cancer treatment, due to the moderate to high prevalence of deglutition disorders in these population. Given the high complication rate and adverse impact on quality of life, it is critical to minimize dysphagia and its sequelae.
Study limitations
This SR had some limitations. The data collected for the parameters analyzed were not from the same number of studies or the same periods after cancer treatment. This is even more evidenced by the lack of data for swallowing parameters according to the topography, treatment type, and/or stage of HNC. There was also high heterogeneity among all quantitative analyses. These factors together limit the quality of evidence collected and analyzed in this review.
Conclusions
The frequency of parameters or complications associated with deglutition disorders was highest in the period immediately following cancer treatment (less than 6 months post-treatment), except for pharyngeal residue, which increased from 6 to 12 months post-treatment. The latter swallowing complication had a high frequency in all post-treatment periods analyzed. One of the most serious complications of deglutition disorders, aspiration, had a frequency of 28.6% for the period less than 3 months post-treatment and 28.2% for the period more than 3 months post-treatment. The evidence presented in the literature highlights the need for additional longitudinal studies with follow-up periods longer than 12 months. In addition, assessment of deglutition parameters using imaging exams with global scales or uniformity in the parameters collected is needed, as well as classification according to type of HNC, different cancer stages, and treatment modality.
References
Kraaijenga SAC, van der Molen L, Van Den Brekel MWM, Hilgers FJM (2014) Current assessment and treatment strategies of dysphagia in head and neck cancer patients: a systematic review of the 2012/13 literature. Curr Opin Support Palliat Care 8:152–163
Huang SH, O'Sullivan B (2013) Oral cancer: current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal 18:233–240
Cohen EE, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS, Stein KD, Lyman GH, Pratt-Chapman ML (2016) American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin 66:203–239
Murphy BA, Gilbert J (2009) Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol 19:35–42
Kraaijenga SA, Oskam IM, Van Son RJ et al (2016) Assessment of voice, speech, and related quality of life in advanced head and neck cancer patients 10-years+ after chemoradiotherapy. Oral Oncol 55:24–30
Lazarus CL (2009) Effects of chemoradiotherapy on voice and swallowing. Curr Opin Otolaryngol Head Neck Surg 17:172–178
Park SS, Choi SH, Hong JA, Hong YH, Jeong NG, Lee SY, Sung MW, Hah JH (2016) Validity and reliability of the Korean version of the Speech Handicap Index in patients with oral cavity cancer. Int J Oral Maxillofac Surg 45:433–439
García-Peris P, Parón L, Velasco C, de la Cuerda C, Camblor M, Bretón I, Herencia H, Verdaguer J, Navarro C, Clave P (2007) Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr 26:710–717
Shune SE, Karnell LH, Karnell MP, Van Daele DJ, Funk GF (2012) Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck 34:776–784
Frowen JJ, Perry AR (2006) Swallowing outcomes after radiotherapy for head and neck cancer: a systematic review. Head Neck 28:932–944
Wall LR, Ward EC, Cartmill B, Hill AJ (2013) Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia 28:481–493
Erkal EY, Canoğlu D, Kaya A et al (2014) Assessment of early and late dysphagia using videofluoroscopy and quality of life questionnaires in patients with head and neck cancer treated with radiation therapy. Radiat Oncol 9:137
Patterson JM, McColl E, Carding PN, Hildreth AJ, Kelly C, Wilson JA (2014) Swallowing in the first year after chemoradiotherapy for head and neck cancer: clinician- and patient-reported outcomes. Head Neck 36:352–358
Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA (2014) Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia 29:223–233
Serel S, Demir N, Karaduman AA, Cengiz M, Yakut Y (2013) Head and neck cancer: changes in artrokinematic parameters of neck and swallowing function after radiotherapy. UHOD 27:97–103
Son YR, Choi KH, Kim TG (2015) Dysphagia in tongue cancer patients. Ann Rehabil Med 39:210–217
Van der Molen L, Heemsbergen WD, de Jong R et al (2013) Dysphagia and trismus after concomitant chemo-intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol 106:364–369
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med 151:264–269
Porto de Toledo I, Pantoja LLQ, Luchesi K, Assad DX, De Luca Canto G, Guerra ENS (2017) Deglutition disorders as a consequence of cancer therapies: a systematic review and meta-analysis. PROSPERO CRD42017067837. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067837. Accessed 17 November 2017
Greenhalgh T, Peacock R (2005) Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 331:1064–1065
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210
Joanna Briggs Institute (2014) Joanna Briggs institute reviewers’ manual: 2014 edition. The systematic review of prevalence and incidence data. The University of Adelaide: The Joanna Briggs Institute p 37
Deeks JJ, Bossuyt PM, Gatsonis C, eds (2009) Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration
Higgins JPT, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available from: www.cochrane-handbook.org
Agarwal J, Palwe V, Dutta D, Gupta T, Laskar SG, Budrukkar A, Murthy V, Chaturvedi P, Pai P, Chaukar D, D’Cruz AK, Kulkarni S, Kulkarni A, Baccher G, Shrivastava SK (2011) Objective assessment of swallowing function after definitive concurrent (chemo)radiotherapy in patients with head and neck cancer. Dysphagia 26:399–406
Cartmill B, Cornwell P, Ward E, Davidson W, Porceddu S (2012) A prospective investigation of swallowing, nutrition, and patient-rated functional impact following altered fractionation radiotherapy with concomitant boost for oropharyngeal cancer. Dysphagia 27:32–45
Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ, Miller AE, Teknos TN, Chepeha DB, Hogikyan ND, Terrell JE, Wolf GT (2002) Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 53:23–28
Graner DE, Foote RL, Kasperbauer JL, Stoeckel RE, Okuno SH, Olsen KD, Sabri AN, Maragos NE, Cha SS, Sargent DJ, Strome SE (2003) Swallow function in patients before and after intra-arterial chemoradiation. Laryngoscope 113:573–579
Kotz T, Costello R, Li Y, Posner MR (2004) Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck 26:365–372
Ku PK, Yuen EH, Cheung DM, Chan BY, Ahuja A, Leung SF, Tong MC, van Hasselt A (2007) Early swallowing problems in a cohort of patients with nasopharyngeal carcinoma: symptomatology and videofluoroscopic findings. Laryngoscope 117:142–146
Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M (2000) Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res 43:1011–1023
Logemann JA, Rademaker AW, Pauloski BR, Lazarus CL, Mittal BB, Brockstein B, MacCracken E, Haraf DJ, Vokes EE, Newman LA, Liu D (2006) Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck 28:64–73
Logemann JA, Pauloski BR, Rademaker AW et al (2008) Swallowing disorders in the first year after radiation and chemoradiation. Head Neck 30:148–158
O'Connell DA, Rieger J, Harris JR et al (2008) Swallowing function in patients with base of tongue cancers treated with primary surgery and reconstructed with a modified radial forearm free flap. Arch Otolaryngol Head Neck Surg 134:857–864
Xinou E, Chryssogonidis I, Kalogera-Fountzila A, Panagiotopoulou-Mpoukla D, Printza A (2018) Longitudinal evaluation of swallowing with videofluoroscopy in patients with locally advanced head and neck cancer after chemoradiation. [published ahead of print March 23, 2018]. Dysphagia. https://doi.org/10.1007/s00455-018-9889-4
Kronenberger MB, Meyers AD (1994) Dysphagia following head and neck cancer surgery. Dysphagia 9:236–244
Haidich AB (2010) Meta-analysis in medical research. Hippokratia 14:29–37
Rinkel RN, Verdonck-de Leeuw IM, Doornaert P, Buter J, de Bree R, Langendijk JA, Aaronson NK, Leemans CR (2016) Prevalence of swallowing and speech problems in daily life after chemoradiation for head and neck cancer based on cut-off scores of the patient-reported outcome measures SWAL-QOL and SHI. Eur Arch Otorhinolaryngol 273:1849–1855
Bhattacharyya N (2014) The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg 151:765–769
Hiss SG, Postma GN (2003) Fiberoptic endoscopic evaluation of swallowing. Laryngoscope 113:1386–1393
Murray J, Langmore SE, Ginsberg S, Dostie A (1996) The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia 11:99–103
Neubauer PD, Hersey DP, Leder SB (2016) Pharyngeal residue severity rating scales based on fiberoptic endoscopic evaluation of swallowing: a systematic review. Dysphagia 31:352–359
Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MWS, Holsinger FC (2012) Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer 118:5793–5799
Huang TL, Chien CY, Tsai WL et al (2016) Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 38:1026–1032
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Isabela Porto de Toledo is supported by CNPq (Brazilian National Council for Scientific and Technological Development). Letícia Lopes Quirino Pantoja was supported by CAPES (Coordination for the Improvement of Higher Education Personnel), Ministry of Education, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Porto de Toledo, I., Pantoja, L.L.Q., Luchesi, K.F. et al. Deglutition disorders as a consequence of head and neck cancer therapies: a systematic review and meta-analysis. Support Care Cancer 27, 3681–3700 (2019). https://doi.org/10.1007/s00520-019-04920-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04920-z