Abstract
Purpose
A clinical care pathway for pain management in a palliative care unit was studied with outcomes related to patients, physicians, and health care service. Mandatory use of patient-reported outcome measures (PROMs) and physician-directed decision support (DS) were integrated parts of the pathway.
Methods
Adult cancer patients with pain intensity (PI) ≥ 5 (NRS 0–10) at admission were eligible. The patients reported average and worst PI at admission, day four, and discharge. The physicians completed the DS at admission and day four. The DS presented potential needs for treatment changes based on pain severity and pathophysiology. The physicians reported treatment changes due to input from the DS system. The two primary outcomes were average and worst PI changes from admission to discharge. Hospital length of stay (LOS) was registered.
Results
Of 52 included patients, 41 were discharged alive. For those, the mean average PI at admission and at discharge was 5.8 and 2.4, respectively, a reduction of 3.4 points (CI 95% 2.7–4.1). The corresponding worst pain intensities were 7.9 and 3.8, a reduction of 4.1 points (CI 95% 3.4–4.8). The physicians completed DS forms for all patients. Fifty-five percent (CI 95% 41–69) of the patients had pain intervention changes based on the DS. A significant reduction in LOS (4.4 days, CI 95% 0.5–8.3) was observed during the study period.
Conclusions
The interventions were implemented according to the intentions and PI was reduced as hypothesized. For evaluation of generalizability, the interventions should be studied in other settings and with a controlled design.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer pain is undertreated, and deficiencies in cancer pain assessment and management may contribute to this lack of success [1,2,3]. The assessment of pain and response to pain management involves the patient, the health care professional, and their interaction [4]. Pain assessment by patient self-report, including a measure for treatment satisfaction, is recommended [1, 5, 6]. The use of standardized patient-reported outcomes has shown validity and reliability [6, 7]. However, a single measurement of patient-reported pain intensity (PI) alone, whether reported within the time frame “now” or as a pain average over the past 24 h, provides the physician with limited knowledge to guide the need for on demand (PRN) pain medication [6]. Information on patient satisfaction with pain control also must be collected [5].
A successful pain treatment is dependent on the physician’s responsiveness to patient input on pain descriptors [4]. Analgesic treatment is potentially effective in most cases [1], and recommendations and guidelines for cancer pain treatment are published [8, 9]. Furthermore, insight on pain etiology and pathophysiological mechanisms may provide additional information to optimize the pain treatment [5]. Without updated knowledge on alternatives for pain therapy such as the use of opioids [8], radiotherapy for painful bone metastases [10], and use of specific adjuvant drugs for neuropathic pain and visceral pain [11, 12], the physician might underuse available options for adequate pain management.
Despite the evidence for both improved symptom control and overall survival by systematic monitoring [13, 14], and the evidence for pain treatment based on the etiological pain classification described in the 11th revision of the World Health Organization’s International Classification of Diseases (ICD-11) [15, 16], there is a gap between available knowledge and real-world practice [1]. The systematic checklist approach used in aviation for decades may also in healthcare ensure that acknowledged standards are applied for every patient, every time [17]. Clinical care pathways are structured plans which detail essential steps in patient treatment, intended to optimize clinical outcomes and efficiency [18]. A care pathway aims to link evidence-based guidelines and clinical expertise and is a suitable method to implement structured pain assessment and checklists into clinical practice [18,19,20].
We hypothesized significant improvements in pain control if patients systematically registered patient-reported outcome measures (PROMs) and if the physicians applied an evidence-based decision support (DS). Elements from implementation research, which addresses both individual and system factors, were applicable to monitor the process [21].
Within the framework of a health care improvement project [22], a prospective intervention study was conducted in a specialized palliative care unit. The intervention was based upon a care pathway structure and included systematic and repeated use of PROMs and mandatory use of physician-directed DS [19]. The overall aim of the study was to investigate the effects and use of the intervention. The two primary outcomes were average and worst PI reductions from admission to discharge. In addition, the number of eligible patients included and reporting PROMs, if and how the physicians used and based their decision-making on the PROMs and DS, and development in hospital length of stay (LOS) during the study period, were secondary outcomes.
Methods
Context
The study was designed as a phase II interventional prospective uncontrolled trial, where the intervention represented measures to accomplish pain treatment according to recommended standards. The Regional Committee for Medical and Health Research Ethics classified the project as quality assurance (2016/548), without the need for expressive informed consent from the patients. The Data Protection Supervisor at St. Olavs Hospital, Trondheim University Hospital, Norway, endorsed the study.
As part of the routine symptom screening, PI is assessed for all patients admitted to the Palliative Care Unit, Cancer Clinic, St. Olavs Hospital, Trondheim University Hospital. Patients with a pain score ≥ 5 on the 11-point numeric rating scale (NRS 0–10) at admittance are in specific need of attention, as their pain is more intense than “mild” [23, 24]. In the period September 2016 to March 2017, all patients with locally advanced and/or metastatic cancer and with a pain score ≥ 5 (NRS 0–10) on admittance were screened for inclusion in the study. Patients < 18 years of age, patients with severe cognitive impairment, patients admitted for planned radiotherapy, and patients unwilling or unable to fill in symptom self-assessment reports were excluded.
Interventions
PROMs were collected at admission, at day four of the hospital stay, and at planned discharge. If needed, assistance from a health care professional was offered. The patients rated the average PI in the past 48 h (NRS 0–10), the worst PI in the past 24 h (NRS 0–10), and the degree of treatment satisfaction with both the around the clock (ATC) and the PRN pain medication (NRS 0–10, 10 representing completely satisfied) [5, 25]. Finally, the patients were asked whether they reported pain flares and requested extra pain medication for such pain (NRS 0–10, 10 representing every time), and if not so, reasons why.
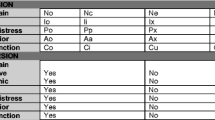
The physicians had access to the collected PROMs when presented with a DS paper form. The DS was filled in by the physicians at admission and at day four of the hospital stay. It was formulated as ten questions with the response options “yes,” “no,” and “uncertain.” By nature, the DS represented “reminders” on possible needs for changes in opioid dose, administration route or opioid rotation, or needs for additional treatment for neuropathic, visceral, or bone pain. The complete DS is presented in Table 3. In addition, the physicians were asked to report whether the pain treatment was changed based on the PROMs and/or the DS.
The regular staff of physicians at the palliative care unit, including the first and second author, conducted the treatment.
Primary outcome measures
Comparison of patient-reported average PI and worst PI at admission and discharge, respectively, were primary outcomes. A PI difference of two points (NRS 0–10) was considered clinically relevant for both primary outcomes [26, 27].
Secondary outcome measures
The number of patients with PI ≥ 5 (NRS 0–10) at admittance, the number of eligible patients included in the study, and the number of patients formally reporting PROMs were secondary patient-related outcomes.
The number of physicians who filled in the DS at admission and at day four was a secondary physician-related outcome. Further physician-related outcomes were the percentages of treatment revisions based on the PROMs and DS at admission, respectively, and changes in the percentage of treatment revisions based on DS information during the study period. Finally, to which degree the physician-reported need for treatment changes at admission was verified when the patient charts were searched for actual treatment changes at discharge, also constituted a secondary physician-related outcome measure.
Besides the secondary outcomes related to the patients and the physicians, change in LOS during the study period was a secondary health care service-related outcome.
Analysis
Recently published research reported a standard deviation (SD) of 2.1 for average PI and an SD of 2.7 for the worst PI for cancer in-patients [28]. Power analysis based on two primary outcomes (reduction in average and worst PI), an SD of 2.7 and an alpha error of 0.025, indicates that a one-sided paired t test carried out on 40 patients will have a minimum power of 0.9 to detect a two-point (NRS 0–10) pre-post PI difference, allowing for repeated measurements correlation of 0.1 or higher. As varying and high attrition rates are reported in supportive care and palliative oncology trials [29], the study was run until the necessary number of consecutive patients with complete data was obtained.
Patients who died during the hospital stay resulted in missing data. Single imputations with last value carried forward were performed for the patients with missing data. Afterwards, the mean average PI and mean worst PI at discharge for all included patients were computed for comparison with the complete cases. The subgroup not able to fill in the PROMs constituted patients in need of end-of-life care, and they were not included in the subsequent effect outcome analyses.
For the patients discharged alive, mean pain intensities at admission and discharge were compared using a paired sample t test.
The number of patients filling in PROMs at admission, at day four of the hospital stay and at planned discharge, were compared to the number of available patients at the respective points of time, and completion rates were calculated.
The completion rate of the DS forms by the physicians at admission and at day four was computed. The percentages of physician-reported treatment changes based on PROMs and DS at admission, respectively, were calculated with 95% confidence intervals (CI). In addition, the percentages of physician-reported treatment changes based on the DS were computed for patients enrolled early, in the mid-phase, and late in the study period. Differences between patients enrolled early versus those enrolled late were tested using independent samples t test and z test for independent proportions, for continuous and binary variables, respectively. Finally, the percentage of concordance between the physician-reported need for treatment changes at admission and documented treatment changes recorded from the medical charts was calculated for each item in the DS. For these calculations, DS responses were dichotomized into “yes” and “no/uncertain,” and treatment changes were dichotomized into “increased” and “decreased/unchanged.”
LOS was reported for patients enrolled early, in the mid-phase, and late in the study period. The difference between early and late enrolment was calculated with 95% CI.
Results
Patient characteristics
From September 2016 to March 2017, 246 patients were admitted to the Palliative Care Unit, Cancer Clinic, St. Olavs Hospital, Trondheim University Hospital. Fifty-two patients with PI ≥ 5 (NRS 0–10) at admission were included in the study, and basic patient characteristics at admission are presented in Table 1. Mean LOS was 10.6 days for the 52 included patients. The reasons for exclusion are listed in Fig. 1. Data registrations at discharge were available for 41 patients.
Pain registrations with imputations for incomplete cases
At admission, for all 52 included patients, the mean average PI in the past 48 h and mean worst PI in the past 24 h were 5.9 and 7.8 (NRS 0–10), respectively. At discharge, with last value carried forward imputations in the 11 patients who died during the hospital stay, mean average PI in the past 48 h and mean worst PI in the past 24 h were 3.0 and 4.3 (NRS 0–10), respectively.
Primary outcomes
For the 41 patients discharged alive, mean average PI in the past 48 h at admission and at discharge were 5.8 and 2.4 (NRS 0–10), respectively. There was a reduction in average PI during the hospital stay of 3.4 points (CI 95% 2.7–4.1, p = 0.00) (Fig. 2). For the same group of patients, mean worst PI in the past 24 h at admission and at discharge were 7.9 and 3.8 (NRS 0–10), respectively. There was a reduction in worst PI during the hospital stay of 4.1 points (CI 95% 3.4–4.8, p = 0.00) (Fig. 2).
Secondary outcomes
In the study period, 22% (54/246) of the admitted patients had PI ≥ 5 (NRS 0–10). Only two eligible patients were not included (Fig. 1). All 52 included patients reported PROMs at admission, and all 46 and all 41 available patients reported PROMs at day four and at discharge, respectively.
DS forms were filled in by the physicians for all 52 and for all 46 available patients at admission and day four, respectively. For 80% (95% CI 69–90%) of the patients, the physicians reported pain intervention revisions at admission based on the PROMs. For 55% (95% CI 41–69%) of the patients, the physicians reported pain intervention revisions at admission based on DS information. The percentages of treatment changes based on the DS throughout the study period are displayed in Table 2. The increase in physician-reported treatment changes based on the DS, for patients enrolled early versus late in the study period, from less than 50% to approximately 70% of the patients, was not statistically significant (p = 0.17). The percentages of concordance between the physician-reported need for treatment changes at admission (collected from the DS forms), and documented treatment changes made during the hospital stay (collected from the charts) are displayed for each item in the DS in Table 3, which also shows selected treatment measures taken during the stay. Besides the 98% concordance for neuraxial pain management in 4% of the patients, the concordance was highest for the DS on ATC opioid dose based on PIs, and for the DS on bone, visceral, and neuropathic pain. The concordance was lowest for the DS on specific treatment for tumor edema and the need for opioid rotation.
Comparing the first third and the last third of the enrolled patients, mean LOS were 12.9 days and 8.5 days, respectively (Table 2). There was a significant reduction in LOS of 4.4 days (CI 95% 0.5–8.3 days, p = 0.03) from patients enrolled early to late in the study period.
Discussion
The use of standardized and repeated PROMs and DS showed effect in the current study. The reduction in average and worst PIs was in the range of three to four points (NRS 0–10), combined with a significant reduction in LOS during the study period. Both the PROMs and the DS were used, and for approximately half of the patients, the physicians reported treatment changes based on the DS.
Appraisal of methods
Randomized trials provide robust evidence about intervention effects [30]. However, studies with observational designs are often used to measure the effectiveness of an intervention in “real-world” scenarios [31]. Despite high compliance with the study protocol and substantial pain reduction during the hospital stay, the current study provides no certain inference of causality between the intervention and the effect. Lack of information on pre-study treatment results and no comparison group contribute to this feature. However, we observed that the patients and the physicians filled in the PROMs and the DS and that pain treatment was changed based on the PROMs for three quarters of the patients and on DS for half of the patients, respectively. These observations ensure that the interventions were applied.
The open-label, one-group study design opens for systematic errors, including bias and confounding [32]. The protocol patients represent a selection of the patients with pain admitted to a palliative care unit, which may limit the generalizability of the results. However, in a “real-world” scenario, these are the patients with the greatest need for improved pain management. The present study showed a large positive effect size. These findings might be interpreted that the intervention works in “real life,” but further studies are needed in order to confirm the results. Furthermore, the generalizability of the results may be limited by the single-center design in a specialized palliative care unit. The complexity of the palliative care given in a specialized hospital unit may influence both the intervention and the outcome and represent confounding factors [32].
Comparison with previous work
Cancer pain treatment according to guidelines has proven efficacy for decades [33]. In addition, treatment algorithms based on guidelines for cancer pain management, and educational interventions promoting their implementation, have resulted in reduced PI [34,35,36]. Still, a recent study combining computerized assessment and DS did not improve cancer pain management [37]. That study provided specific suggestions for treatment modifications. However, 15 years ago, ten “commandments” for effective clinical DS was published, emphasizing the importance of clinicians’ autonomy [38]. The DS in the present study consisted of ten questions, encouraging the clinician to reflect on potential needs for changes in pain treatment. The results indicate that the clinicians addressed the questions raised in the DS and followed up the identified need for treatment changes.
Simplicity and user-friendliness are success criteria for clinical DS systems [38, 39]. In the current study, the concordance between the need for treatment changes indicated in the DS and the documented treatment changes made during the hospital stay was more than 80% for six questions. Neuraxial pain management was, despite the high concordance, a relevant treatment option for only a small proportion of the patients, and therefore perhaps not needed in a general DS for all pain patients. The questions on average and worst PI at admission, with respect to ATC opioid dose at discharge, both yielded high concordance. These findings are in line with previous research, supporting both alternatives [6, 9]. For simplicity, one of the questions might be chosen in routine clinical use. To ensure the necessary focus on treatment needs based on pain etiology and pathophysiology [16], the DS might be supplemented by three questions on the need for radiotherapy for painful bone metastases, indication for adjuvant drugs for neuropathic, pain, and indication for specific treatment for visceral pain.
The concept of personalized symptom goals is suggested incorporated in future symptom assessment [6]. A personalized pain goal represents the PI level the patient would be comfortable with and [6], compared with actual PI, provides indirect information on treatment satisfaction with the ongoing pain management. In addition, the degree of treatment satisfaction with specific pain management includes some evaluation of side effects. We found a concordance between the physician-interpreted PROM on pain treatment satisfaction at admission and documented change in ATC opioid dose during the hospital stay of almost 80%. Based on this finding, and previous research underlining the importance of treatment satisfaction when evaluating pain control [5], both patient-reported treatment satisfaction and personalized symptom goals might be relevant in pain assessments.
Knowledge deficiencies are demonstrated in several areas of cancer pain management, including opioid titration, opioid rotation, and cancer pain pathophysiology [2, 4]. Among oncologists, knowledge on cancer pain management and adherence to pain treatment guidelines vary widely [2, 3]. Surprisingly enough, also in the study conducted in a specialized palliative care unit, the physicians reported pain intervention revisions at admission based on DS information for half the patients. The findings may implicate that a DS both may guide health care personnel with limited knowledge and act as a reminder for health care providers more competent in cancer pain treatments. The current study is an example of the latter situation, where a simple intervention, when used rigorously, yielded large reductions in PI. The effects from the clinical pathway for health care workers less proficient in pain treatment must be observed in future studies. The potential for improvement will depend on different factors ranging from pain assessment and response to patient input, to knowledge on cancer pain management, adherence to clinical practice guidelines, and available treatment options.
A care pathway should ideally include explicit statements of the goals and key elements of care, the roles, and sequence of the activities of the multidisciplinary team, and the monitoring and evaluation of variances and outcomes [19]. The aim of the care pathway is to organize and standardize the care process in order to maximize patient outcomes and improve organization efficiency [40]. Within the existing framework of the multifaceted care process in a specialized palliative care unit, the systematic approach represented by the intervention, triggered by NRS ≥ 5 on PI and consisting of the planned use of PROMs and DS, is a “micro” care pathway for a sub-cohort of patients admitted to a specialized palliative care unit. In addition to better pain management, the use of clinical care pathways has been associated with a reduction in LOS [41], as also shown in our study.
Bundles of care are evidence-based practices grouped together to encourage delivery of evidence-based care [42]. They usually consist of a small, straightforward set of practices, that when performed collectively and reliably, improve patient outcomes [43]. The repeated assessments and pain treatment reminders in the present study, based on clinical guidelines, also could be considered a bundle of care. As in studies on care bundles, drawing conclusions about which combinations of individual interventions that maximize the effect is difficult [43].
Implications and further work
Our findings support the importance of systematic and repeated cancer pain assessment and treatment according to existing guidelines [4, 6, 7]. In addition, the study demonstrated high compliance with the interventions. Before definitive conclusions can be drawn, the interventions should be studied in a randomized trial. For generalizability, the intervention also must show effect in other patient populations. A systematic review, based on 148 randomized trials, reported improved “health care process measures” due to clinical DS systems [44]. However, with physician response rates to the presented prompts as low as 50% [45], compliance with the DS is a challenge that needs to be addressed. Another challenge in clinical settings is developing DS simple enough for practicality and complex enough for effect.
Conclusions
In a specialized palliative care unit, and studied in a single sample with an open-label design, standardized assessments and physician-directed DS were used and PI reductions were demonstrated. LOS was reduced during the study period. The interventions should be studied in other settings and with a controlled design.
References
Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, Apolone G (2014) Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 32(36):4149–4154. https://doi.org/10.1200/JCO.2014.56.0383
Breuer B, Chang VT, Von Roenn JH, von Gunten C, Neugut AI, Kaplan R, Wallenstein S, Portenoy RK (2015) How well do medical oncologists manage chronic cancer pain? A national survey. Oncologist 20(2):202–209. https://doi.org/10.1634/theoncologist.2014-0276
te Boveldt N, Vernooij-Dassen M, Besse K, Vissers K, Engels Y (2015) Adaptation of an evidence-based clinical practice guideline in cancer pain management by medical oncologists: a case vignette study. Support Care Cancer 23(5):1409–1420. https://doi.org/10.1007/s00520-014-2472-0
Kwon JH (2014) Overcoming barriers in cancer pain management. J Clin Oncol 32(16):1727–1733. https://doi.org/10.1200/JCO.2013.52.4827
Lohre ET, Klepstad P, Bennett MI, Brunelli C, Caraceni A, Fainsinger RL, Knudsen AK, Mercadante S, Sjogren P, Kaasa S European Association for Palliative Care Research N (2016) From “Breakthrough” to “Episodic” Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey Toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J Pain Symptom Manag 51(6):1013–1019. https://doi.org/10.1016/j.jpainsymman.2015.12.329
Hui D, Bruera E (2017) The Edmonton symptom assessment system 25 years later: past, present, and future developments. J Pain Symptom Manag 53(3):630–643. https://doi.org/10.1016/j.jpainsymman.2016.10.370
Richardson LA, Jones GW (2009) A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol 16(1):55
Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G, European Palliative Care Research C, European Association for Palliative C (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 13(2):e58–e68. https://doi.org/10.1016/S1470-2045(12)70040-2
Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, Ripamonti CI, Committee EG (2018) Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol 29(Supplement_4):iv166–iv191. https://doi.org/10.1093/annonc/mdy152
Rich SE, Chow R, Raman S, Liang Zeng K, Lutz S, Lam H, Silva MF, Chow E (2018) Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol 126(3):547–557. https://doi.org/10.1016/j.radonc.2018.01.003
Brunelli C, Bennett MI, Kaasa S, Fainsinger R, Sjogren P, Mercadante S, Lohre ET, Caraceni A, European Association for Palliative Care Research N, International Association for the Study of Pain Cancer Pain Special Interest G (2014) Classification of neuropathic pain in cancer patients: a Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain 155(12):2707–2713. https://doi.org/10.1016/j.pain.2014.09.038
Ferguson HJ, Ferguson CI, Speakman J, Ismail T (2015) Management of intestinal obstruction in advanced malignancy. Ann Med Surg (Lond) 4(3):264–270. https://doi.org/10.1016/j.amsu.2015.07.018
Strasser F, Blum D, von Moos R, Cathomas R, Ribi K, Aebi S, Betticher D, Hayoz S, Klingbiel D, Brauchli P, Haefner M, Mauri S, Kaasa S, Koeberle D, Swiss Group for Clinical Cancer R (2016) The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Ann Oncol 27(2):324–332. https://doi.org/10.1093/annonc/mdv576
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318(2):197–198. https://doi.org/10.1001/jama.2017.7156
Bennett MI, Eisenberg E, Ahmedzai SH, Bhaskar A, O'Brien T, Mercadante S, Krcevski Skvarc N, Vissers K, Wirz S, Wells C, Morlion B (2018) Standards for the management of cancer-related pain across Europe-A position paper from the EFIC Task Force on Cancer Pain. Eur J Pain. https://doi.org/10.1002/ejp.1346
Bennett MI, Kaasa S, Barke A, Korwisi B, Rief W, Treede RD, Pain ITCC (2019) The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain 160(1):38–44. https://doi.org/10.1097/j.pain.0000000000001363
Clay-Williams R, Colligan L (2015) Back to basics: checklists in aviation and healthcare. BMJ Qual Saf 24(7):428–431. https://doi.org/10.1136/bmjqs-2015-003957
Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, Snow P, Kugler J (2010) Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 3:CD006632. https://doi.org/10.1002/14651858.CD006632.pub2
Schrijvers G, van Hoorn A, Huiskes N (2012) The care pathway: concepts and theories: an introduction. Int J Integr Care 12(Spec Ed Integrated Care Pathways):e192
Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, Brunelli C, Caraceni A, Cervantes A, Currow DC, Deliens L, Fallon M, Gomez-Batiste X, Grotmol KS, Hannon B, Haugen DF, Higginson IJ, Hjermstad MJ, Hui D, Jordan K, Kurita GP, Larkin PJ, Miccinesi G, Nauck F, Pribakovic R, Rodin G, Sjogren P, Stone P, Zimmermann C, Lundeby T (2018) Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 19(11):e588–e653. https://doi.org/10.1016/S1470-2045(18)30415-7
Theobald S, Brandes N, Gyapong M, El-Saharty S, Proctor E, Diaz T, Wanji S, Elloker S, Raven J, Elsey H, Bharal S, Pelletier D, Peters DH (2018) Implementation research: new imperatives and opportunities in global health. Lancet 392(10160):2214–2228. https://doi.org/10.1016/S0140-6736(18)32205-0
Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D (2016) SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 25(12):986–992. https://doi.org/10.1136/bmjqs-2015-004411
Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC (2013) Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manag 45(6):1083–1093. https://doi.org/10.1016/j.jpainsymman.2012.06.007
Chow E, Ding K, Parulekar WR, Wong RK, van der Linden YM, Roos D, Hartsell WF, Hoskin P, Wu JS, Nabid A, Ong F, van Tienhoven G, Babington S, Demas WF, Wilson CF, Brundage M, Zhu L, Meyer RM (2015) Revisiting classification of pain from bone metastases as mild, moderate, or severe based on correlation with function and quality of life. Support Care Cancer. https://doi.org/10.1007/s00520-015-2957-5
Lohre ET, Hjermstad MJ, Brunelli C, Knudsen AK, Kaasa S, Klepstad P (2018) Pain intensity factors changing breakthrough pain prevalence in patients with advanced cancer: a secondary analysis of a cross-sectional observational international study. Pain Ther 7(2):193–203. https://doi.org/10.1007/s40122-018-0107-8
Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL (2000) Defining the clinically important difference in pain outcome measures. Pain 88(3):287–294
Chow E, Hoskin P, Mitera G, Zeng L, Lutz S, Roos D, Hahn C, van der Linden Y, Hartsell W, Kumar E (2012) Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 82(5):1730–1737. https://doi.org/10.1016/j.ijrobp.2011.02.008
Thronaes M, Raj SX, Brunelli C, Almberg SS, Vagnildhaug OM, Bruheim S, Helgheim B, Kaasa S, Knudsen AK (2016) Is it possible to detect an improvement in cancer pain management? A comparison of two Norwegian cross-sectional studies conducted 5 years apart. Support Care Cancer 24(6):2565–2574. https://doi.org/10.1007/s00520-015-3064-3
Hui D, Glitza I, Chisholm G, Yennu S, Bruera E (2013) Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 119(5):1098–1105. https://doi.org/10.1002/cncr.27854
Welch VA, Norheim OF, Jull J, Cookson R, Sommerfelt H, Tugwell P, Equity C, Boston Equity S (2017) CONSORT-Equity 2017 extension and elaboration for better reporting of health equity in randomised trials. BMJ 359:j5085. https://doi.org/10.1136/bmj.j5085
Anglemyer A, Horvath HT, Bero L (2014) Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 4:MR000034. https://doi.org/10.1002/14651858.MR000034.pub2
Kacha AK, Nizamuddin SL, Nizamuddin J, Ramakrishna H, Shahul SS (2018) Clinical study designs and sources of error in medical research. J Cardiothorac Vasc Anesth. https://doi.org/10.1053/j.jvca.2018.02.009
Walker VA, Hoskin PJ, Hanks GW, White ID (1988) Evaluation of WHO analgesic guidelines for cancer pain in a hospital-based palliative care unit. J Pain Symptom Manag 3(3):145–149
Du Pen SL, Du Pen AR, Polissar N, Hansberry J, Kraybill BM, Stillman M, Panke J, Everly R, Syrjala K (1999) Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. J Clin Oncol 17(1):361–370. https://doi.org/10.1200/JCO.1999.17.1.361
Du Pen AR, Du Pen S, Hansberry J, Miller-Kraybill B, Millen J, Everly R, Hansen N, Syrjala K (2000) An educational implementation of a cancer pain algorithm for ambulatory care. Pain Manag Nurs 1(4):116–128. https://doi.org/10.1053/jpmn.2000.19333
Fallon M, Walker J, Colvin L, Rodriguez A, Murray G, Sharpe M, Edinburgh Pain A, Management Tool Study G (2018) Pain management in cancer center inpatients: a cluster randomized trial to evaluate a systematic integrated approach-the Edinburgh pain assessment and management tool. J Clin Oncol 36(13):1284–1290. https://doi.org/10.1200/JCO.2017.76.1825
Raj SX, Brunelli C, Klepstad P, Kaasa S (2017) COMBAT study - computer based assessment and treatment - a clinical trial evaluating impact of a computerized clinical decision support tool on pain in cancer patients. Scand J Pain 17:99–106. https://doi.org/10.1016/j.sjpain.2017.07.016
Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B (2003) Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 10(6):523–530. https://doi.org/10.1197/jamia.M1370
Blum D, Raj SX, Oberholzer R, Riphagen II, Strasser F, Kaasa S, Euro Impact EIMPCRT (2015) Computer-based clinical decision support systems and patient-reported outcomes: a systematic review. Patient 8(5):397–409. https://doi.org/10.1007/s40271-014-0100-1
Lawal AK, Rotter T, Kinsman L, Machotta A, Ronellenfitsch U, Scott SD, Goodridge D, Plishka C, Groot G (2016) What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Med 14:35. https://doi.org/10.1186/s12916-016-0580-z
Furuhata H, Araki K, Ogawa T, Ikeda M (2017) Effect on completion of clinical pathway for improving clinical Indicator: cases of hospital stay, mortality rate, and comprehensive-volume ratio. J Med Syst 41(12):206. https://doi.org/10.1007/s10916-017-0857-6
Hellyer TP, Ewan V, Wilson P, Simpson AJ (2016) The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc 17(3):238–243. https://doi.org/10.1177/1751143716644461
Ospina MB, Mrklas K, Deuchar L, Rowe BH, Leigh R, Bhutani M, Stickland MK (2017) A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax 72(1):31–39. https://doi.org/10.1136/thoraxjnl-2016-208820
Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, Samsa G, Hasselblad V, Williams JW, Musty MD, Wing L, Kendrick AS, Sanders GD, Lobach D (2012) Effect of clinical decision-support systems: a systematic review. Ann Intern Med 157(1):29–43. https://doi.org/10.7326/0003-4819-157-1-201207030-00450
Carroll AE, Anand V, Downs SM (2012) Understanding why clinicians answer or ignore clinical decision support prompts. Appl Clin Inform 3(3):309–317. https://doi.org/10.4338/ACI-2012-04-RA-0013
Acknowledgments
The Norwegian Cancer Society supported this study. All authors had full access to all of the data in the study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Løhre, E.T., Thronæs, M., Brunelli, C. et al. An in-hospital clinical care pathway with integrated decision support for cancer pain management reduced pain intensity and needs for hospital stay. Support Care Cancer 28, 671–682 (2020). https://doi.org/10.1007/s00520-019-04836-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04836-8