Abstract
Purpose
To determine if time to antibiotics (TTA) improves outcomes of hospital length of stay, admission to the intensive care unit, and 30-day mortality in adult patients with febrile neutropenia.
Methods
This retrospective cohort study evaluated the impact of time to antibiotic, in the treatment of febrile neutropenia, on hospital length of stay, admission to the intensive care unit, and 30-day mortality. Cases included were patients 18 years or older hospitalized with febrile neutropenia from August 1, 2006 to July 31, 2016. To adjust for other characteristics associated with hospital length of stay, admission to the intensive care unit, and 30-day mortality, a multivariate analysis was performed.
Results
A total of 3219 cases of febrile neutropenia were included. The median hospital length of stay was 7.0 days (IQR 4.1–13.3), rate of intensive care unit admission was 13.6%, and 30-day mortality was 6.6%. Multivariate analysis demonstrated time to antibiotics was not associated with hospital length of stay but was associated with admission to the intensive care unit admission and 30-day mortality. Delays in time to antibiotic of up to 3 hours did not impact outcomes.
Conclusions
A shorter time to antibiotic is important in treatment of febrile neutropenia; however, moderate delays in antibiotic administration did not impact outcomes. Further investigation is needed in order to determine if other indicators of infection, in addition to fever, or other supportive management, in addition to antibiotics, are indicated in the early identification and management of infection in patients with neutropenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Infections are a leading cause of mortality in patients with malignancy [1, 2], particularly among those with neutropenia [2,3,4]. Neutropenia not only impairs a patient’s ability to resist and respond to infection, but also alters the presentation of infection with subtle or no localizing signs [2,3,4,5,6]. Fever is often the first, and occasionally the only indication of infection in patients with neutropenia [4]. The high mortality and complex presentation have led to guidelines advocating early empiric antimicrobial therapy in febrile neutropenia, which has resulted in a decrease in infection-related mortality [5, 7,8,9,10].
Guidelines continue to recommend early empiric antimicrobials in the setting of febrile neutropenia, most recently focusing on time to antibiotic (TTA) [9,10,11,12,13,14,15,16,17]. This TTA recommendation has largely been based on results from the Surviving Sepsis Campaign [13, 16]. In the febrile neutropenic population specifically, there is limited and conflicting evidence that TTA impacts outcomes [7, 8, 10, 13, 15,16,17,18]. While some studies have suggested TTA improves hospital length of stay [17] and mortality [8], several other studies have failed to demonstrate a similar association [7, 17,18,19,20].
As TTA continues to be emphasized in present guidelines, TTA has also become a marker of quality of care [20,21,22]. However, when TTA is targeted and improved, outcomes have not changed [20]. Therefore, we evaluated a large population of patients with malignancy-associated febrile neutropenia in order to determine if TTA improves outcomes at our institution.
Methods
We obtained Institutional Review Board approval at Mayo Clinic (Rochester, MN) to use the existing electronic medical record (EMR) of patients that have given previous authorization for research (IRB 15-006458). We developed an electronic search strategy using the Advanced Cohort Explorer (ACE), a data query tool which provides access to clinical notes, laboratory values, and patient vital information, including temperature recordings.
We based our definition of febrile neutropenia from the Infectious Disease Society of America (IDSA) guideline. We included adult patients with an absolute neutrophil count (ANC) 500 cells/mm3 or less who were evaluated from August 1, 2006 through July 31, 2016. Our electronic algorithm searched these patients for the clinical diagnosis of febrile neutropenia made within 48 hours of laboratory confirmation of neutropenia. Noting that if patients had an ANC > 500 cells/mm3 at time of fever, but were expected to have a drop in ANC to 500 cells/mm3 or less within 48 hours, did fulfill IDSA criteria for febrile neutropenia. We used clinical documentation rather than documented objective fever (temperature > 38.3 °C at any single time or a temperature of 38 °C or greater sustained for a 1 hour period), as many patients had reported fever prior to presentation [23].
Two physicians (LD and UD) reviewed a convenience sample of 100 medical records of patients, with an ANC of 500 cells/mm3 or less. The sample was selected using a random number generator. The reviewers evaluated clinical notes, laboratory values, and temperature recordings to decide if a patient met criteria for febrile neutropenia at the time of the diagnosis as identified by the electronic algorithm. The inter-rater agreement (LD and UD) was calculated using the intra-class correlation coefficient and was 0.96. The agreement between the electronic algorithm and reviewer (LD) was 0.91 with 96% agreement. Thus validated, we used the electronic algorithm to identify the cohort of patients with febrile neutropenia.
The remaining data was collected using ACE in conjunction with Datamart, an electronic database housing comprehensive data including medications and time of administration [24]. Time of fever was defined as the time of first documented temperature of 38.3 °C, first documented temperature of at least 38 °C sustained for a 1-hour period, or time of triage with presentation fever [15]. For simplicity, only the first episode of febrile neutropenia treated during hospitalization was included. Patients determine to be low risk, as determined by Multinational Association for Supportive Care in Cancer (MASCC) score, and treated with oral antibiotics were excluded [7, 25]. Antibiotics used to determine time of administration were those included in the analysis and were those recommended for high-risk patients per the IDSA guidelines: piperacillin/tazobactam, cefepime, meropenem, aztreonam, or aminoglycosides. The difference in time of fever to time of antibiotic administration was used to calculate TTA.
Other variables were then collected. Vital signs, including systolic blood pressure and pulse rate, were those available at time of fever. Laboratory values, including blood culture results and ANC, were those taken closest to time of fever and limited to within 24 hour of time of fever. Treatment with growth factor support was determined by documentation of recent administration of medication or active prescription. Underlying malignancy was determined by documented diagnosis in clinical notes. Clinical diagnosis of infection was manually confirmed by using a combination of culture results, imaging, and provider documentation of infection. The Charlson Comorbidity Index, a well-validated prognostication tool for longitudinal studies of survival, was electronically calculated and used to determine overall comorbidity burden [25]. The attending physician determined admission to the ICU. Admission, transfer, and discharge data was collected. We excluded patients not admitted to the hospital during treatment of febrile neutropenia, patients without malignancy, patients who were already on appropriate antibiotics at time of fever, and patients who were transferred to our hospital with diagnosis of febrile neutropenia (where TTA was unknown) [3]. Finally, we excluded patients who did not receive antibiotics within 48 hours of fever.
Statistical analysis
TTA was categorized based on the IDSA guideline recommendation available at the time of the study, with time to antibiotic of 0–2 hours as the reference [3]. The 3-hour, 6-hour, and 24-hour references were based on the Surviving Sepsis Campaign sepsis bundles available during this study period [16]. Patient demographics and clinical characteristics were summarized using descriptive statistics. Covariate distributions were compared using chi-square and Kruskal-Wallis tests when appropriate.
Our primary outcome was 30-day mortality. Univariable and multivariable logistic regressions were used to determine the association between time to antibiotic and 30-day mortality. Survival over the 30-day period was depicted using a Kaplan-Meier curve. Predictors of secondary outcomes such as hospital length of stay (LOS) and intensive care unit (ICU) admission were also examined using multivariable linear and logistic regressions, respectively. Variables were included if they demonstrated statistical significance in univariate analysis and/or they were deemed to be clinically significant. All tests were 2-sided, and performed using SAS software, Version 9.4, and STATA software, Version 14. [26]
Results
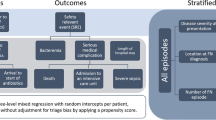
We identified a total of 10,155 adult patients with an absolute neutrophil count of 500 cells/mm3 or less, seen at our facility between August 1, 2006 and July 31, 2016. We excluded 7552 patients for the following reasons: refusal to consent (N = 313), lack of fever (N = 5118), outpatient treatment of febrile neutropenia (N = 50), no underlying malignancy (N = 548), already on antibiotics at time of fever (N = 602), low-risk MASCC score and did not receive antibiotics recommended by guidelines for high-risk neutropenic fever (N = 807), or receipt of treatment at an outside institution prior to arrival (N = 114). A total of 2603 patients with 3219 hospitalizations were included in the analysis (Fig. 1).
We found that 50.6% of patients received antibiotics within the recommended 2 hours from time of fever (Table 1). The majority of patients had hematologic malignancy (lymphoma 51.4%, leukemia 36.2%). Patients had a clinical diagnosis of infection in 45.1% of cases of febrile neutropenia and 48.8% of these patients were found to have a blood stream infection. Overall, the rate of blood stream infection was 22% with gram positive being the most common (10.4%), followed by gram negative (6.8%), polymicrobial (4.6%), fungal (0.1%), and mycobacterial (0.0%) (Table 1). The median hospital length of stay after time of fever was 7.0 days (IQR 4.1–13.3), rate of ICU admission was 13.6%, and 30-day mortality was 6.6%.
Thirty-day mortality increased from 5% if TTA was within 2 hours to 13% if TTA was greater than 24 h (P < 0.01). After adjusting for confounders, longer TTA was not associated with increased hospital LOS. When compared to patients who received antibiotics within 2 hours of fever, patients who received antibiotics 2–3 hours, 3–6 hours, and 24–48 hours after time of fever had a hospital length of stay coefficient value of 0.93 (P = 0.73), 1.71 (P = 0.38) and − 4.57 (P = 0.15) respectively. After adjusting for confounders, a longer TTA was significantly associated with increased risk of admission to ICU and 30-day mortality (Table 2). Factors associated with increased mortality included age, Charlson Comorbidity Index, shock index, ANC less than 500 cell/mm3 at time of fever (noting expected drop to ANC < 500 within 48 hours as IDSA criteria), prior admission for neutropenic fever, blood culture result, and clinical diagnosis of infection. When compared to patients who received antibiotics within 2 hours of fever, patients who received antibiotics 3–6 hours and 24–48 hours after time of fever had odds ratio for mortality of 1.57 (P = 0.04) and 2.08 (P = 0.02), respectively (Table 2). Receipt of antibiotics 6–24 hours after fever was not associated with a significant increased risk of mortality compared to receipt within 2 hours of fever (Table 2). Kaplan-Meier survival of patients, differentiated by time to antibiotics, is depicted in Fig. 2.
Discussion
To our knowledge, this is the largest study on TTA in febrile neutropenia [3, 8, 17,18,19,20,21]. We found that a shorter TTA is associated with the rate of ICU admission and 30-day mortality. The association with TTA was most notable at 3 hours, suggesting that moderate delays, of up to 3 hours, in antibiotics were tolerated [17, 21, 27]. This finding is different than previous studies and guideline recommendations [8, 23, 28]. However, based on this study, it is unclear why moderate delays in TTA were tolerated.
While other studies have demonstrated an association between TTA and composite outcomes of ICU admission and mortality in patients with febrile neutropenia, to our knowledge, there is only one study that has demonstrated an association between TTA and mortality alone [7,8,9, 17, 29]. This was a prospective study by Rosa et al., in which a cohort of hospitalized patients with febrile neutropenia received a grouped intervention of immediate empiric antibiotics and evaluation by the medical fast response team [8]. With this grouped intervention, Rosa et al. demonstrated that a shorter TTA was associated with improved mortality, with each hour delay in TTA increasing 28-day mortality by 18% [8]. Rosa et al. also showed the mortality benefit persisted in patients who had an infection not susceptible to the initial early empiric antibiotics, suggesting time to effective antibiotics did not impact mortality [8]. This finding is consistent with previous studies on TTA, which have shown that mortality is not negatively impacted by moderate delays in time to effective antibiotics [17, 30, 31]. Nevertheless, Rosa et al. demonstrated that, regardless of bacterial susceptibilities, mortality was still improved with their grouped intervention. Therefore, the additional intervention of immediate evaluation by the medical fast response team may have been what impacted mortality, not TTA alone [8]. This would also be consistent with evidence in patients with severe sepsis and septic shock where grouped interventions, not antibiotics alone, have been shown to improve mortality [22, 32]. For this reason, our ability to demonstrate that TTA did impact rates of ICU admission and mortality, while moderate delays were tolerated, could be due to the additional time provided for other important interventions to be implemented. This may also be why previous studies on TTA in febrile neutropenia have been inconsistent, as other interventions have not been implemented and/or measured. Therefore, while early empiric antibiotics are important, they may not be the only important intervention needed early in patients with febrile neutropenia.
Conversely, fever may not be the only indicator for infection in patients with neutropenia. We found that patients with shock index > 1.0 had an increased rate of ICU admission and a similar risk of mortality as patients with long delays in antibiotics. This is consistent with previous evidence which has shown that after adjusting for confounders, including shock, that TTA is no longer predictive of mortality [17]. Furthermore, patients with neutropenia and infection, with or without fever, have been shown to have higher mortality in the setting of severe sepsis or shock [7, 33, 34]. There is also evidence suggesting that in the setting of neutropenia and infection, patients who are afebrile have worse outcomes than febrile patients [35]. IDSA guideline recommends that patients with neutropenia who develop new signs or symptoms of infection, with or without fever, should be started on the same early empiric antibiotic coverage as high-risk patients with fever [3]. Therefore, fever may not be the only or most sensitive indicator for infection in patients with neutropenia.
Additionally, fever may no longer be specific for infection in the setting of malignancy-related neutropenia. Previously, infection was identified in the majority of patients with febrile neutropenia; however, in recent studies, including this study, infection is identified in the minority of patients with febrile neutropenia [1, 8, 29, 33, 35]. Recent studies have also shown the overall mortality in febrile neutropenic patients has declined, while infection-related mortality remains high [1,2,3]. In particular, we found the rate of ICU admission and mortality associated with infection was still higher than the rate of ICU admission and mortality associated with long delays in antibiotic administration. Therefore, the association between TTA and rate of ICU admission or mortality may have been diluted by cases in which there was no infection for antibiotics to impact. As a result, we were only able to detect a difference after moderate delays in antibiotics. Likewise, moderate delays in antibiotics may not have impacted outcomes because the moderate delay was tolerated by the majority of patients who had no infection identified. Therefore, fever may not be sufficiently specific for infection in patients with neutropenia.
Limitations
This study was limited due to the retrospective data collection. Additionally, at our institution, most of our hematology and oncology patients are hospitalized in the campus without a progressive care unit; therefore, patients’ ICU admission may have reflected such things as telemetry needs rather than need for advanced critical care. This may have impacted our ability to detect an association between TTA and rate of ICU admission. Furthermore, we have dedicated outpatient services specific for the hematology and oncology patients who require daily monitoring; therefore, we are able to continue treatments offered in the hospital in the outpatient setting. This may have impacted our inability to detect an association between TTA and hospitalization length of stay. These differences may also be due to specifics of our local practices, as this was a single-center study.
We chose to evaluate TTA as a categorical variable, in order to evaluate the TTA values use in quality metrics and in accordance with guideline recommendations at the time of this study. However, using TTA as a categorical variable, rather than a continuous variable, potentially limited our power and increased our chance of a type II error.
Conclusion
Time to antibiotics is important in treatment of febrile neutropenia; however, moderate delays in antibiotic administration did not impact outcomes. Currently, patients with neutropenia and infection continue to have management focused on TTA; however, the findings of our study warrant further investigation into the role of supportive care other than antibiotics and defining additional indicators of infection other than fever in management of patients with neutropenia and infection.
Abbreviations
- ACE:
-
Advanced Cohort Explorer
- ANC:
-
Absolute neutrophil count
- EMR:
-
Electronic medical record
- ICU:
-
Intensive care unit
- IDSA:
-
Infectious Disease Society of America
- LOS:
-
Length of stay
- MASCC:
-
Multinational Association for Supportive Care in Cancer
- TTA:
-
Time to antibiotic
References
Hersh EM, Bodey GP, Nies BA, Freireich EJ (1965) Causes of death in acute leukemia: a ten-year study of 414 patients from 1954-1963. JAMA 193:105–109
Curtin JA, Marshall BD Jr (1962) Use of antibiotics in cancer and leukemia. J Chronic Dis 15:713–718
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of A (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93
DiNubile MJ (1995) Fever and neutropenia: still a challenge. Contemp Intern Med 7:35–37 41-35
Pizzo PA (1993) Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med 328:1323–1332
Sickles EA, Greene WH, Wiernik PH (1975) Clinical presentation of infection in granulocytopenic patients. Arch Intern Med 135:715–719
Butts AR, Bachmeier CC, Dressler EV, Liu M, Cowden A, Talbert J, Adams VR (2017) Association of time to antibiotics and clinical outcomes in adult hematologic malignancy patients with febrile neutropenia. J Oncol Pharm Pract 23:278–283
Rosa RG, Goldani LZ (2014) Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother 58:3799–3803
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F, Group EGW (2010) Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol 21(Suppl 5):v252–v256
Zuckermann J, Moreira LB, Stoll P, Moreira LM, Kuchenbecker RS, Polanczyk CA (2008) Compliance with a critical pathway for the management of febrile neutropenia and impact on clinical outcomes. Ann Hematol 87:139–145
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Keng MK, Sekeres MA (2013) Febrile neutropenia in hematologic malignancies. Curr Hematol Malig Rep 8:370–378
Baden LR, Bensinger W, Angarone M, Casper C, Dubberke ER, Freifeld AG, Garzon R, Greene JN, Greer JP, Ito JI, Karp JE, Kaul DR, King E, Mackler E, Marr KA, Montoya JG, Morris-Engemann A, Pappas PG, Rolston K, Segal B, Seo SK, Swaminathan S, Naganuma M, Shead DA, National Comprehensive Cancer N (2012) Prevention and treatment of cancer-related infections. J Natl Compr Cancer Netw 10:1412–1445
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL, Young LS (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines C, American Association of Critical-Care N, American College of Chest P, American College of Emergency P, Canadian Critical Care S, European Society of Clinical M, Infectious D, European Society of Intensive Care M, European Respiratory S, International Sepsis F, Japanese Association for Acute M, Japanese Society of Intensive Care M, Society of Critical Care M, Society of Hospital M, Surgical Infection S, World Federation of Societies of I, Critical Care M (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Perron T, Emara M, Ahmed S (2014) Time to antibiotics and outcomes in cancer patients with febrile neutropenia. BMC Health Serv Res 14:162
Keng MK, Thallner EA, Elson P, Ajon C, Sekeres J, Wenzell CM, Seastone DJ, Gallagher EM, Weber CM, Earl MA, Mukherjee S, Pohlman B, Cober E, Foster VB, Yuhas J, Kalaycio ME, Bolwell BJ, Sekeres MA (2015) Reducing time to antibiotic administration for febrile neutropenia in the emergency department. J Oncol Pract 11:450–455
Szwajcer D, Czaykowski P, Turner D (2011) Assessment and management of febrile neutropenia in emergency departments within a regional health authority-a benchmark analysis. Curr Oncol 18:280–284
Ko BS, Ahn S, Lee YS, Kim WY, Lim KS, Lee JL (2015) Impact of time to antibiotics on outcomes of chemotherapy-induced febrile neutropenia. Support Care Cancer 23:2799–2804
Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE (2015) The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med 43:1907–1915
Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, Kuderer NM, Langston AA, Marr KA, Rolston KV, Ramsey SD (2013) Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31:794–810
Best JT, Frith K, Anderson F, Rapp CG, Rioux L, Ciccarello C (2011) Implementation of an evidence-based order set to impact initial antibiotic time intervals in adult febrile neutropenia. Oncol Nurs Forum 38:661–668
Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O (2010) Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc 85:247–254
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
StataCorp (2015) Stata Statistical Software: Release 14. In: Editor (ed)^(eds) Book Stata Statistical Software: Release 14. StataCorp LP, City
Fletcher M, Hodgkiss H, Zhang S, Browning R, Hadden C, Hoffman T, Winick N, McCavit TL (2013) Prompt administration of antibiotics is associated with improved outcomes in febrile neutropenia in children with cancer. Pediatr Blood Cancer 60:1299–1306
Gonzalez-Barca E, Fernandez-Sevilla A, Carratala J, Salar A, Peris J, Granena A, Gudiol F (1999) Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis 18:539–544
Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K, Paesmans M (2007) Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 30(Suppl 1):S51–S59
Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, Kline JA, Jones AE, Emergency Medicine Shock Research N (2011) Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 39:2066–2071
Cho SY, Lee DG, Choi SM, Kwon JC, Kim SH, Choi JK, Park SH, Park YJ, Choi JH, Yoo JH (2013) Impact of vancomycin resistance on mortality in neutropenic patients with enterococcal bloodstream infection: a retrospective study. BMC Infect Dis 13:504
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative G (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, Lemiale V, Seguin A, Darmon M, Schlemmer B, Azoulay E (2012) Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med 40:43–49
Elting LS, Rubenstein EB, Rolston KV, Bodey GP (1997) Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis 25:247–259
Strojnik K, Mahkovic-Hergouth K, Novakovic BJ, Seruga B (2016) Outcome of severe infections in afebrile neutropenic cancer patients. Radiol Oncol 50:442–448
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We obtained Institutional Review Board approval at Mayo Clinic (Rochester, MN) to use the existing electronic medical record (EMR) of patients that have given previous authorization for research (IRB 15-006458).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
There are no duplicate/previous publications from this study.
Electronic supplementary material
ESM 1
(DOCX 1187 kb)
Rights and permissions
About this article
Cite this article
Daniels, L.M., Durani, U., Barreto, J.N. et al. Impact of time to antibiotic on hospital stay, intensive care unit admission, and mortality in febrile neutropenia. Support Care Cancer 27, 4171–4177 (2019). https://doi.org/10.1007/s00520-019-04701-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04701-8