Abstract
Purpose
To assess the effectiveness and safety of a product containing diosmin, coumarin, and arbutin (Linfadren®) in addition to complex decongestive therapy (CDT) on the management of patients with a breast cancer-related lymphedema (BCRL).
Methods
Fifty outpatients (average age of 56.2 ± 2.7 years, range 28–71) with a BCRL were enrolled for this study. Patients were randomly assigned (1:1 ratio) to receive either CDT consisting of skin care, manual lymphatic drainage, remedial exercises, and elastic compression garment (control group, n = 25) or CDT plus Linfadren® (study group, n = 25). Patients were evaluated before and after treatment and 3 months after the end of treatment. Primary outcomes were reduction of upper limb excess volume (EV) and percentage reduction of excess volume (%REV). Secondary outcomes were improvement in Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire, and patient’s perception of treatment effectiveness (PPTE).
Results
Addition of Linfadren® to CDT yielded an additional reduction of primary outcomes both after treatment (EV, − 521 ml vs. − 256 ml, P < 0.0001; %REV, − 66.4% vs. − 34%, P = 0.02) and at 3-month follow-up (EV, − 59 ml vs. + 24 ml, P < 0.0001; %REV, − 73.6% vs. − 31.4%, P = 0.004). Moreover, statistically significant differences were found between the two groups for the secondary outcomes after treatment (QuickDASH, P = 0.006; PPTE, P = 0.03) and at 3-month follow-up (QuickDASH, P = 0.006; PPTE, P = 0.02). No patient showed adverse events.
Conclusions
Linfadren® in addition to CDT was a safe and effective therapy for reducing BCRL and was better than CDT alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer-related lymphedema (BCRL) is a common complication after surgery for breast cancer [1, 2].
BCRL develops due to interruption of the axillary lymphatic system, leading to regional or generalized accumulation of lymph fluid in the interstitial space of the upper limb [3, 4]. The incidence of BCRL rises from 3 to 15% after sentinel node biopsy, to 10–20% after complete axillary dissection, and 30–50% with subsequent radiotherapy [5, 6].
BCRL can cause physical impairments, psychological distress, and suboptimal health-related quality of life [4, 7]. Consequently, appropriate treatments are important for reducing these complications. Complex decongestive therapy (CDT), which is a two-phase program, is considered the best practice in the management of lymphedema [8, 9]. Nevertheless, CDT often produces inconsistent limb volume reduction [10, 11], is time consuming, and costly, and its success is dependent upon trained and experienced therapists [12], and compliance of patients is not always optimal, particularly for the self-administered phase 2 of treatment [13]. Moreover, CDT interventions primarily remove fluid congestion from the tissues, without completely reducing the concentration of proteins in the interstitium. [14]. The persistence of a high concentration of proteins in the interstitium maintains high oncotic pressure, which creates a vicious circle with an incomplete resolution of lymphedema [14].
Therefore, a pharmacological treatment that could reduce the stagnant protein excess and fluid congestion from the interstitium, improving and helping to maintain the results obtained with CDT, it should be of interest in the management of BCRL [14, 15].
Benzopyrones (e.g., diosmin and coumarin) are a group of drugs that have been found to be successful in treating lymphedema [14,15,16,17,18,19,20,21,22], particularly when combined with CDT [16, 18, 21]. Their use in the treatment of a high-protein chronic edema such as lymphedema has the advantage of acting not only on the fluid component of lymphedema but also on the excess interstitial proteins, whose removal occurs by increasing proteolysis by macrophages. The removal of the excess interstitial proteins will lead to further and longer lasting reduction of lymphedema. In the same manner, inflammation and consequent fibrotic transformations, as well as possible bacterial infections, will be reduced. [14].
Although benzopyrones have been acknowledged as a potential component of the multidisciplinary, therapeutic approach to treating patients with BCRL [23], a recent review failed to establish any conclusions due to the poor quality of the analyzed trials [24], suggesting that there is a need to conduct randomized clinical trials on this topic.
Accordingly, we undertook this clinical study to investigate the effectiveness and safety of a mixture of 200 mg of diosmin, 30.6 mg of coumarin, and 3.7 mg of arbutin, commercialized in Italy with the name of Linfadren® (OMEGA PHARMA Srl, Cantù (CO), Italy), when used in addition to CDT in patients with BCRL.
Materials and methods
Study design
This prospective, pragmatic, open-label, randomized, active-controlled, and assessor- and statistician-blinded clinical study was carried out in an outpatient rehabilitation center. The study was approved by the local institutional review board of the University of L’Aquila (Prot. 30262) and was performed according to the Declaration of Helsinki, and the International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines.
All patients received complete information regarding the study and gave their written informed consent before entry into the study.
Subjects
Women aged 18 years and older, who developed stage I to III BCRL according to the International Society of Lymphology [25], were eligible for this study. The inclusion criterion was a confirmed diagnosis of BCRL. Exclusion criteria were: primary lymphedema; bilateral lymphedema; pulmonary edema; congestive heart failure; history of inflammatory, metabolic, or neuropathic arthropathies; hepatic diseases; bleeding disorders; severe gastrointestinal diseases; the presence of any contraindications limiting clinical evaluation; and/or the use of the treatment modalities and/or drugs used in this study to manage the BCRL.
Among 78 patients with BCRL examined, 21 meet the exclusion criteria (9 inflammatory arthropathies, 3 congestive heart failure, 1 syringomyelia with upper limb involvement, 2 pulmonary edema, 4 hepatic diseases, 2 bleeding disorders), and 7 refused to participate. Therefore, 50 patients (all women) with an average age of 56.2 ± 2.7 years (range 28–71) were enrolled in this study.
Patients’ demographic characteristics including age, BMI, type of surgery, chemotherapy, radiation, time since surgery, affected upper limb, dominant upper limb side, concomitant medications, circumference and volume of both upper limbs, and scheduled patient self-administered questionnaires were collected at the baseline visit.
Table 1 summarizes demographic data, which were similar and without statistically significant differences between groups.
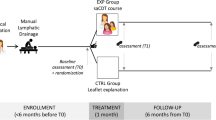
Patients were randomly assigned, using a computer-based 1:1 randomization scheme and sealed envelopes, to receive either CDT (control group, N = 25) or same CDT plus Linfadren® (study group, N = 25). A flow chart of the study is illustrated in Fig. 1.
Patients of both groups were treated in two phases: first intensive phases, with CDT conducted once a day for 5 days a week for 2 weeks, and second extensive phase, with compression garments, remedial exercises, and skin care for 4 weeks.
CDT in our study included skin care, manual lymphatic drainage, remedial exercises, and compression garments [26] instead of multi-layer bandaging. Despite its proven effectiveness, the use of multi-layer bandaging is expensive, requiring daily bandaging resources, and often is not well tolerated by patients [27, 28], also worsening the upper limb function [29]. Whereas, compression garments, which have been proven to be effective in the management of secondary lymphedema [30], are easy to use, do not interfere with continuing daily activities, are well tolerated by patients increasing their compliance to treatment [31]. The pressure of garments was chosen on the basis of lymphedema severity [26], expressed as percentage of excess limb volume [25], as follows: 14–18 mmHg in mild lymphedema, 20–25 mm/Hg in moderate and severe lymphedema, 25–30 mmHg in selected cases of severe lymphedema [26].
A compression garment was applied after manual lymphatic drainage, before beginning upper limb exercises and maintained 23 h/daily.
Manual lymphatic drainage was performed according to the Vodder’s method [32] for 45 min. Remedial exercises were performed in the following order: warm up by active mobilization of the upper limb joints at a moderate pace for 5 min; deep abdominal breathing exercise 3–5 times; range of motion exercises particularly for the shoulder joint for 20 times; decongestive exercise for the arm and shoulder, elbow, and wrist 10–15 repetitions of each.
Patients in the study group received the same treatment of the control group plus an adjunctive supplementation of Linfadren®. Patients were instructed to consume Linfadren® without food twice a day (6-hourly: 11 a.m. and 5 p.m.) for 2 weeks, and once a day for 4 weeks. Due to the pragmatic nature of this study, Linfadren® was prescribed by physicians and purchased from chemists by patients. To improve adherence in medication intake, we asked patients to self-compile a daily diary. Moreover, during the first 2 weeks, the drug intake was reminded to patients during physiotherapy sessions, while in the following 4 weeks, patients were periodically contacted by phone [33]. The study procedure was conducted by trained physiotherapists and a physician who were not involved in any way in the scheduled assessments of patients, as well as in data elaboration and analysis.
Outcome measures
Patients were assessed before and after the first phase of treatment and 3 months after the end of the first phase of treatment (follow-up) by an independent treatment–blinded physician.
Primary outcome measures
The primary outcome measures of this study were (1) reduction of the mean value of excess volume (EV) of the affected limb (VAL) relative to the unaffected limb (VUL) and calculated as (VAL − VUL) and (2) percentage reduction of excess limb volume (%REV) calculated as: %REV = (VAL Post − VAL Pre)/EV × 100, from before treatment to after treatment and to the 3-month follow-up period.
All volumes were derived by modified truncated cone method of circumference measurements on both upper limbs. Upper limb circumferences were measured using a cloth measuring tape at four levels in both upper limbs: metacarpophalangeal joints, wrist joint, 15 cm distal and 10 cm proximal to the lateral epicondyle. During measurement procedures, patients were comfortably seated with their upper limbs at their sides and their elbows straight while the circumference measurements were taken. Two measurements were taken and their mean was used. The circumference differences (in centimeters) between the two upper limbs were calculated at all four levels. The derived final volume was determined by adding the separate volumes of the sections together. Every calculation was squared, and then all measurements for that arm were totaled and divided by π. The recorded values were used to calculate absolute volume of affected (VAL) and unaffected (VUL) limbs using the following formula:
where V is the volume of the segment, C1 and C2 are the circumferences at the ends of the segment, and h is the distance between them [34, 35].
Secondary outcome measures
The secondary end points were the upper limb disability and patient self-perceived treatment effectiveness. To evaluate the upper limb disability, we chose the Quick Disabilities of Arm, Shoulder and Hand (QuickDASH) questionnaire [36], which is a valid and reliable instrument for measurement of upper limb disability in breast cancer survivors [37]. It consists of 11 core items designed to measure physical function, symptoms and social function, and 2 optional items (musical or sports performance and work), which generate a disability score, ranging from 0 (no disability) to 100 (severe disability) [36, 37].
To evaluate patient self-perceived treatment effectiveness, a 5-point Likert scale ranging from “extremely effective” to “not at all effective” was chosen. Success rates were calculated by dichotomizing responses. Patients who referred to treatment effectiveness as “extremely effective” or “very effective” were counted as successes, whereas patients who referred to treatment effectiveness as “somewhat effective,” “poorly effective,” or “not at all effective” were counted as failures.
Tolerability of Linfadren®
To evaluate the tolerability of the Linfadren® during the course of treatment, patients underwent before and after treatment a complete laboratory examination, including hematology, blood chemistry, and urinalysis. Patients also underwent a clinical examination to record vital signs and were asked to record all the adverse effects they observed.
Statistical analysis
The calculation of the number of patients was based on the primary outcomes. For the volume at the end of treatment, it was assumed for this study that the patients in the control group would have a mean reduction of 200 mL, and the patients in the study group a mean reduction of 300 mL, with a common standard deviation of 100 mL. On this basis, and assuming a level of significance of 5% and a power of 80%, the necessary sample size was determined to be 21 patients per group. Assuming a dropout of 15%, 25 patients per group were required. Statistical analyses were performed using the MedCalc, version 11.1.1.0 for Windows (MedCalc Software, Mariakerke, Belgium) All analyses were performed according to the principle of intention-to-treatment, in which patients with missing data were counted as treatment failures.
A two-sample unpaired t test and chi-square (χ2) test were applied to compare the differences of continuous and categorical variables, respectively.
A two-way analysis of variance (ANOVA) with group (study versus control) as the between-patient factor and time (before-after-follow-up) as the within-subjects factor was used to assess the presence of significant differences between the study and control groups and within each group before and after treatment and at the 3-month follow-up. A Tukey post hoc comparison was used to determine significant differences between mean values when a significant main effect and interaction were found for EV and QuickDASH questionnaire.
For all analyses, two-sided P values less than or equal to 0.05 were considered significant.
Results
The baseline characteristics that were similar and without statistic significance for both groups are shown in Table 1. Patients received the following concomitant medications (no. of patients in study group/control group): antidepressants, anxiolytics/mood stabilizers (4/3), antidiabetics (3/3), antihypertensive (4/5), antiosteoporosis and vitamin D/calcium (10/8), antidyslipidemic (not statins) (5/4), osteoarthritis supplements (12/11), non-steroidal anti-inflammatory (3/2), thyroid hormone replacement therapy (1/2), gastric protection (7/5). All patients were reexamined after the treatment period. At the 3-month follow-up, 1 patient in the study group and 2 patients in the control group were lost, 2 (1 in the study group and 1 in the control group) had received pneumatic compression treatment in another center and 1 because had undergone lymphatic drainage treatment in another center between the end of treatment and follow-up. Nevertheless, based on the intent-to-treat principle, the data for these 3 patients were included in the data analysis.
The lymphedema severity at baseline was 47.8% in the study group and 46.6% in the control group, which was severe lymphedema based on the definition of the International Society of Lymphology [25]. Lymphedema severity improved to minimal lymphedema in the study group both after treatment (16.2%) and at 3-month follow-up (12.6%), whereas lymphedema severity improved to moderate lymphedema in the control group both after treatment (30.5%) and at 3-month follow-up (31.8%).
Primary outcome measures
Two-way ANOVA demonstrated a significant effect of treatment (P ≤ 0.001) and a significant treatment–time interaction (P ≤ 0.001) for EV. Post hoc comparison within each group showed (Table 2) a statistically significant reduction of EV (P < 0.0001) in both groups after treatment. However, at 3-month follow-up, a statistically significant reduction of EV with respect to after treatment was observed in the study group (P = 0.03), but not in the control group, which showed a little although not significant worsening (P = 0.4). Post hoc comparison between groups of the same parameter showed (Table 2) significant differences between the study and control groups both after treatment (P < 0.0001) and at the 3-month follow-up (P < 0.0001).
Chi-square (χ2) test for %REV showed significant differences between the study and control groups after treatment (P = 0.02) and at the 3-month follow-up (P = 0.004) (Table 2). After treatment, %REV was − 66.4% in the study group and − 34% in control group. At 3-month follow-up, although both without statistical significance, an improvement of 10.8% (from − 66.4 to − 73.6%) of %REV is found in the study group, whereas a worsening of 7.6% (from − 34 to − 31.4%) is shown in the control group (Table 2).
Secondary outcome measures
Two-way ANOVA demonstrated a significant effect of treatment (P ≤ 0.001) and a significant treatment–time interaction (P ≤ 0.001) for QuickDASH score. Post hoc comparison within each group showed (Table 3) a statistically significant reduction (P < 0.0001) in both groups. However, at 3-month follow-up, a statistically significant (P = 0.04) improvement was observed in the study group, whereas a near statistically significant (P = 0.06) worsening was observed in the control group.
Fisher’s exact test revealed that the percentage of patients satisfied of the treatment (Likert scale scores of “1” (much improved) or “2” (somewhat improved) (i.e., successful results) was statistically higher in the study group than in the control group both after treatment (P = 0.03) and at 3-month follow-up (P = 0.02). The Likert scores for both groups are shown in Table 4.
Tolerability of Linfadren®
No adverse events were recorded during the study in both groups. The results of the clinical and laboratory examinations revealed no signs of systemic toxicity due to Linfadren® administration (Table 5).
Discussion
BCRL is a very common complication observed in clinical practice in patients who undergo surgery for breast cancer [1, 2]. Although BCRL is incurable, several conservative interventions have been proposed for the control of symptoms, minimize complications, and improve function and quality of life [4].
The results of our randomized trial show that Linfadren® plus CDT yields better results than CDT alone in the management of patients with BCRL. After treatment, we found a significant %REV of approximately 66.4% in patients where Linfadren® was used adjunctively with CDT; patients where CDT was the sole treatment experienced a 34% %REV. It should be noted that at 3-month follow-up, %REV improved in the group treated with Linfadren® plus CDT, while %REV worsened in the group treated with CDT alone. Also for the functional improvement of the affected upper limb, as measured by QuickDASH questionnaire, the greater improvements were seen in those patients where Linfadren® was used additionally with CDT, with respect to patients treated with CDT alone, both at the end of the treatment as well as at 3-month follow-up. It should be noted that at 3-month follow-up, QuickDASH continued to improve significantly in the group treated with CDT plus Linfadren®, while it was worse although not significantly in the group treated with CDT alone.
Better benefits in the patients’ perceived effectiveness of treatment were also seen in patients receiving Linfadren® plus CDT compared with those receiving CDT alone.
Despite a direct comparison of various studies being difficult, our results, in agreement with previous studies where benzopyrones have been used in combination with CDT [14, 16, 18], show that the combination of Linfadren® plus CDT leads to better results than CDT alone in the management of patients with BCRL.
In the literature, the magnitude of the benefit due to the combination of benzopyrones and CDT, with respect to CDT or benzopyrones alone, varies. This might be a result of variation in the drugs and doses used, as well as in the measurement method of volume reduction. For example, Casley-Smith & Casley-Smith [16] report that if benzopyrones are used in combination with CDT, the reduction of edema volume increased from 130 to 200% with respect to CDT alone.
The results observed in our patients treated with CDT alone are similar to previous studies in which patients treated with CDT have achieved a mean reduction of excess arm volume around 25–30% [10, 38, 39].
Also analyzing the studies where benzopyrones are used as the sole treatment of BCRL, the reduction of the excess volume are lower (ranging from 7 to 35%) [14, 20, 40], in comparison to that obtained in our study where Linfadren® was used in combination with CDT.
These findings suggest that the combination of benzopyrones or Linfadren® with CDT is better than CDT or benzopyrones alone in the management of patients with BCRL. Similar suggestions are provided by a pharmacogenomics study [15].
The mechanism by which the main components of Linfadren®, coumarin, diosmin, and arbutin, act to improve the lymphatic disorders still remains poorly understood. It has been postulated that α-benzopyrones (coumarin) activate the proteolytic activity of macrophages, reducing the high concentration of interstitial proteins, which are the most responsible for BCRL [14]. The γ-benzopyrones (diosmin) act on the lymphatic system by increasing lymph oncotic pressure and the frequency and intensity of the contractions of lymphatic vessels, as well as augmenting the total number of functional lymphatic capillaries [20]. Arbutin has diuretic properties that help reduce water tissue retention [41, 42].
In our study therapy with Linfadren® is well tolerated and remarkably free of systemic adverse effects. Regarding the supposed hepatotoxicity of coumarin, no hepatotoxic effect was found in our study at doses of 61.2 mg/day for 2 weeks and 30.6 mg/day for 4 weeks. Analyzing the literature, it would seem that greater toxicity has been found at doses of 400 mg/day [43], while poor and transient effects have been reported at doses of 135 and 90 mg/day [40]. Taken together, the foregoing findings would seem to suggest that the possible adverse effects of coumarin are dose-dependent. Clinical studies have shown that also diosmin has no contraindication even when used at doses of 900 mg/day or concomitantly with other drugs or in elderly people [44]. Similarly for Arbutin, despite long-term use, even at doses of 400–800 mg/day in the treatment of urinary tract infections [42], no cases of toxicity have been reported in humans [42, 45]. Taken together, the foregoing findings would seem to suggest that the possible adverse effects of coumarin are dose-dependent, and regarding diosmin and arbutin, their use in humans do not produce side effects at the recommended doses.
Possible limitations of our study are a small number of patients enrolled, although it did meet the power requirement; the indirect method of volume measurement (truncated cone formula); and the use of the elastic band instead of the multi-layer bandage, already in the first phase of CDT. However, both groups have used the same treatment, and given the pragmatic nature of the study, we have used the treatments that we usually offer to patients in our center; the lack of an untreated control group, which we did not include due to the pragmatic nature of the study; a follow-up period that was not sufficiently long enough to determine the long-term effect of the treatment with CDT plus Linfadren®, and to assess its effects on the long-term quality of life in our patients.
Due to aforesaid limitations, additional studies are needed to confirm these findings. Nevertheless, the differences in scores between the two groups and within the Linfadren® plus CDT group were significant, and the results suggest that the use of Linfadren® in addition to CDT is not only safe but also more effective than CDT alone in the management of patients with BCRL without adverse events.
References
DiSipio T, Rye S, Newman B, Hayes S (2013) Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 14:500–515. https://doi.org/10.1016/S1470-2045(13)70076-7
Omar MT, Shaheen AA, Zafar H (2012) A systematic review of the effect of low-level laser therapy in the management of breast cancer-related lymphedema. Support Care Cancer 20:2977–2984. https://doi.org/10.1007/s00520-012-1546-0
Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS (2009) Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol 7:29–45. https://doi.org/10.1089/lrb.2008.1026
Río-González Á, Molina-Rueda F, Palacios-Ceña D, Alguacil-Diego IM (2018) Living with lymphoedema-the perspective of cancer patients: a qualitative study. Support Care Cancer 26:2005–2013. https://doi.org/10.1007/s00520-018-4048-x
McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ (2008) Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 26:5213–5219. https://doi.org/10.1200/JCO.2008.16.3725
Belmonte R, Messaggi-Sartor M, Ferrer M, Pont A, Escalada F (2018) Prospective study of shoulder strength, shoulder range of motion, and lymphedema in breast cancer patients from pre-surgery to 5 years after ALND or SLNB. Support Care Cancer 26:3277–3287. https://doi.org/10.1007/s00520-018-4186-1
Ridner SH (2005) Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13:904–911. https://doi.org/10.1007/s00520-005-0810-y
Lasinski BB (2013) Complete decongestıve therapy for treatment of lymphedema. Semin Oncol Nurs 29:20–27. https://doi.org/10.1016/j.soncn.2012.11.004
Smile TD, Tendulkar R, Schwarz G, Arthur D, Grobmyer S, Valente S, Vicini F, Shah C (2018) A review of treatment for breast cancer-related lymphedema: paradigms for clinical practice. Am J Clin Oncol 41:178–190. https://doi.org/10.1097/COC.0000000000000355.
Dayes IS, Whelan TJ, Julian JA, Parpia S, Pritchard KI, D'Souza DP, Kligman L, Reise D, LeBlanc L, McNeely ML, Manchul L, Wiernikowski J, Levine MN (2013) Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 31:3758–3763. https://doi.org/10.1200/JCO.2012.45.7192
Javid SH, Anderson BO (2013) Mounting evidence against complex decongestive therapy as a first-line treatment for early lymphedema. J Clin Oncol 31:3737–3738. https://doi.org/10.1200/JCO.2013.51.8373
Fife CE, Davey S, Maus EA, Guilliod R, Mayrovitz HN (2012) A randomized controlled trial comparing two types of pneumatic compression for breast cancer-related lymphedema treatment in the home. Support Care Cancer 20:3279–3286. https://doi.org/10.1007/s00520-012-1455-2
Brown JC, Cheville AL, Tchou JC, Harris SR, Schmitz KH (2014) Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Support Care Cancer 22:135–143. https://doi.org/10.1007/s00520-013-1962-9
Casley-Smith JR, Morgan RG, Piller NB (1993) Treatment of lymphedema of the arms and legs with 5,6 benzo-[alpha]-pyrone. N Engl J Med 329:1158–1163. https://doi.org/10.1056/NEJM199310143291604
Farinola N, Piller N (2005) Pharmacogenomics: its role in re-establishing coumarin as treatment for lymphedema. Lymphat Res Biol 3:81–86. https://doi.org/10.1089/lrb.2005.3.81
Casley-Smith JR, Casley-Smith JR (1996) Treatment of lymphedema by complex physical therapy with and without oral and topical benzopyrones: what should therapists and patients expect. Lymphology 29:76–82
Chang TS, Gan JL, Fu KD, Huang WY (1996) The use of 5,6 benzo-[alpha]-pyrone (coumarin) and heating by microwaves in the treatment of chronic lymphedema of the legs. Lymphology 29:106–111
Casley-Smith JR, Boris M, Weindorf S, Lasinski B (1998) Treatment for lymphedema of the arm--the Casley-Smith method: a noninvasive method produces continued reduction. Cancer 83(suppl 12):2843–2860
Pecking AP, Février B, Wargon C, Pillion G (1997) Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology 48:93–98. https://doi.org/10.1177/000331979704800115
Pecking AP (1995) Evaluation by lymphoscintigraphy of the effect of a micronized flavonoid fraction (Daflon 500 mg) in the treatment of upper limb lymphedema. Int Angiology 14:39–43
Bollinger A, Herrig I, Fischer M, Hoffmann U, Franzeck UK (1995) Intravital capillaroscpy in patients with chronic venous insufficiency and lymphedema: relevance to Daflon 500 mg. Int J Microcirc Clin Exp 15(Suppl 1):41–44
Casley-Smith JR (2000) Changes in the microcirculation at the superficial and deeper levels in lymphoedema: the effects and results of massage, compression, exercise and benzopyrones on these levels during treatment. Clin Hemorheol Microcirc 23:335–343
Li L, Yuan L, Chen X, Wang Q, Tian J, Yang K, Zhou E (2016) Current treatments for breast cancer-related lymphoedema: a systematic review. Asian Pac J Cancer Prev 17:4875–4883. https://doi.org/10.22034/APJCP.2016.17.11.4875.
Badger C, Preston N, Seers K, Mortimer P (2004) Benzo-pyrones for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev 2:CD003140. https://doi.org/10.1002/14651858.CD003140.pub2
International Society of Lymphology (2013) The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology 46:1–11
Framework L (2006) Best practice for the management of lymphoedema. In: International consensus. MEP Ltd, London
Damstra RJ, Brouwer ER, Partsch H (2008) Controlled, comparative study of relation between volume changes and interface pressure under short-stretch bandages in leg lymphedema patients. Dermatol Surg 34:773–778. https://doi.org/10.1111/j.1524-4725.2008.34145.x
Rabe E, Partsch H, Hafner J, Lattimer C, Mosti G, Neumann M, Urbanek T, Huebner M, Gaillard S, Carpentier P (2017) Indications for medical compression stockings in venous and lymphatic disorders: an evidence-based consensus statement. Phlebology [Epub ahead of print] 33:163–184. https://doi.org/10.1177/0268355516689631
King M, Deveaux A, White H, Rayson D (2012) Compression garments versus compression bandaging in decongestive lymphatic therapy for breast cancer-related lymphedema: a randomized controlled trial. Support Care Cancer 20:1031–1036. https://doi.org/10.1007/s00520-011-1178-9
Mahran SA, Moshref SS (2011) The effectiveness of a modified complete decongestive therapy program in the treatment of lymphedema cases. JKAU Med Sci 18:37–51
Cohen SR, Payne DK, Tunkel RS (2001) Lymphedema: strategies for management. Cancer 92:980–987
Kasseroller RG (1998) The Vodder school. the Vodder method Cancer 83(suppl 12):2840–2842
Laufs U, Rettig-Ewen V, Böhm M (2011) Strategies to improve drug adherence. Eur Heart J 32:264–268. https://doi.org/10.1093/eurheartj/ehq297
Koul R, Dufan T, Russell C, Guenther W, Nugent Z, Sun X, Cooke AL (2007) Efficacy of complete decongestive therapy and manual lymphatic drainage on treatment-related lymphedema in breast cancer. Int J Radiat Oncol Biol Phys 67:841–846. https://doi.org/10.1016/j.ijrobp.2006.09.024
Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J (2006) Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther 86:205–214
Beaton DE, Wright JG, Katz JN (2005) Development of the QuickDASH: comparison of three item reduction approaches. J Bone Joint Surg Am 87:1038–1046. https://doi.org/10.2106/JBJS.D.02060
LeBlanc M, Stineman M, DeMichele A, Stricker C, Mao JJ (2014) Validation of quick DASH outcome measure in breast cancer survivors for upper extremity disability. Arch Phys Med Rehabil 95:493–498. https://doi.org/10.1016/j.apmr.2013.09.016
Randheer S, Kadambari D, Srinivasan K, Bhuvaneswari V, Bhanumathy M, Salaja R (2011) Comprehensive decongestive therapy in postmastectomy lymphedema: an Indian perspective. Indian J Cancer 48:397–402. https://doi.org/10.4103/0019-509X.92250
Szuba A, Achalu R, Rockson SG (2002) Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer 95:2260–2267. https://doi.org/10.1002/cncr.10976.
Burgos A, Alcaide A, Alcoba C, Azcona JM, Garrido J, Lorente C, Moreno E, Murillo E, Olsina-Pavia J, Olsina-Kissler J, Samaniego E, Serra M (1999) Comparative study of the clinical efficacy of two different coumarin dosages in the management of arm lymphedema after treatment for breast cancer. Lymphology 32:3–10
Saeed F, Mehjabeen SSK, Noor-Jahan AM (2015) Diuretic & anti-urolithic activity of some crude extracts. Int J Pharm Phyto Pharmacol Res 7:128–131
EMEA, 2017. European Medicines Agency. Assessment report on Arctostaphylos uva-ursi (L.) Spreng., folium. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2017/04/WC500225872.pdf.
Morrison L, Welsby PD (1995) Side-effects of coumarin. Postgrad Med J 71:701–702
Meyer OC (1994) Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 45:579–584. https://doi.org/10.1177/000331979404500614
de Arriba SG, Naser B, Nolte KU (2013) Risk assessment of free hydroquinone derived from Arctostaphylos Uva-ursi folium herbal preparations. Int J Toxicol 32:442–453. https://doi.org/10.1177/1091581813507721
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cacchio, A., Prencipe, R., Bertone, M. et al. Effectiveness and safety of a product containing diosmin, coumarin, and arbutin (Linfadren®) in addition to complex decongestive therapy on management of breast cancer-related lymphedema. Support Care Cancer 27, 1471–1480 (2019). https://doi.org/10.1007/s00520-018-4514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4514-5