Abstract
Purpose
Fosaprepitant improved prevention of chemotherapy-induced nausea and vomiting (CINV) in a randomized, double-blind phase III trial (PN031). This post hoc analysis explored factors that may have influenced response.
Methods
Adult subjects (N = 1000) scheduled to receive non-anthracycline and cyclophosphamide (AC) moderately emetogenic chemotherapy (MEC) on day 1 were randomly assigned 1:1 to a single-dose, 150-mg intravenous fosaprepitant regimen or a control regimen. Both regimens included dexamethasone and ondansetron on day 1, with ondansetron continuing through day 3 in the control arm only. Complete response (CR; no vomiting and no rescue medication) rates in the acute, delayed, and overall phases (0–25, 25–120, and 0–120 h, respectively) were analyzed by chemotherapy type (carboplatin-based vs non-carboplatin-based), chemotherapy duration (single-day vs multiple-day), and baseline characteristics.
Results
Most subjects received single-day chemotherapeutic regimens (70.6%), which were mainly carboplatin-based (67.6%). CR with fosaprepitant was consistent (76–80%) during the delayed and overall phases in carboplatin-based and non-carboplatin-based subgroups and in subgroups receiving single-day or multiple-day MEC regimens. Treatment effects favored fosaprepitant for the carboplatin-based versus the non-carboplatin-based group during the delayed phase (14.1 vs 6.5%; p = 0.06), and for the single-day versus the multiple-day subgroup during the delayed (13.2 vs 3.2%; p = 0.02) and overall phases (12.8 vs 4.0%; p = 0.06).
Conclusions
This exploratory analysis confirms that single-dose fosaprepitant is effective for the prevention of CINV in subjects receiving carboplatin or non-carboplatin in both single- and multiple-day non-AC MEC chemotherapy regimens. This trial is registered at ClinicalTrials.gov, number NCT01594749.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is common with cancer treatment, occurring in 30–90% of people receiving moderately emetogenic chemotherapy (MEC) [1,2,3]. CINV is complicated by chemotherapy sequence, dosing, and multiple-day treatment regimens, along with patient-related risk factors, such as younger age, female sex, history of low alcohol intake, and history of emesis during pregnancy [3, 4]. Therefore, these risk factors should also be considered when developing an antiemetic regimen [3]. Advances in understanding the pathophysiology of CINV have led to new antiemetic therapeutic options. In 2003, approval of the first neurokinin-1 (NK-1) receptor antagonist, oral aprepitant [5], signified an important therapeutic advancement for CINV prophylaxis. In 2008, fosaprepitant dimeglumine, a water-soluble prodrug of aprepitant that rapidly converts to aprepitant after intravenous (IV) administration, was approved [6]. A recent randomized, double-blind phase III trial of 1000 subjects evaluated the efficacy and safety of a single-day, triple-antiemetic fosaprepitant regimen (fosaprepitant, ondansetron, and dexamethasone) for prevention of CINV connected with non-anthracycline and cyclophosphamide (AC)-based MEC [7]. The fosaprepitant regimen was associated with significantly higher rates of complete response (CR; no vomiting or use of rescue medication) in the delayed (25–120 h after initiation of first MEC dose; primary end point) and overall (0–120 h) phases. No significant differences were noted for CR during the acute phase (0–24 h), but the efficacy of both regimens during this phase is highlighted by achievement rates of > 90% in both treatment arms. The fosaprepitant regimen was well tolerated [7]. This was the first well-designed study to evaluate an NK-1 receptor antagonist in a large, well-defined, non-AC MEC population.
The current post hoc analysis was performed to explore factors that might impact the severity of CINV and to assess the ability of a single-day regimen consisting of fosaprepitant 150 mg IV, 5-hydroxytryptamine type 3 (5-HT3) antagonist, and corticosteroid to prevent CINV (as measured by achievement of CR), compared with the standard 3-day antiemetic control regimen. These factors include chemotherapy type (e.g., comparing carboplatin at the upper end of the MEC emetogenic spectrum vs other non-carboplatin MEC therapies) and chemotherapy duration (i.e., comparing single vs multiple-day chemotherapy).
Methods
Study design and subjects
This exploratory analysis was based on data from a global, double-blind, randomized, active-comparator, parallel-group, multicenter, phase III trial (PN031) [7]. Key entry criteria have been reported previously [7]. Briefly, adults (≥ 18 years) with confirmed malignancy, who were treatment-naive to MEC and highly emetogenic chemotherapy (HEC) and scheduled to receive ≥ 1 dose of IV non-AC-based MEC on day 1, were included [7]. Exclusion criteria were vomiting in the 24-h period before day 1; using antiemetic within 48 h before day 1; symptomatic primary or metastatic central nervous system malignancy causing nausea and/or vomiting; and using any dose of cisplatin or other HEC [7]. All eligible patients with information on their chemotherapy regimens (i.e., chemotherapy type and chemotherapy duration) were included in this exploratory analysis. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by ethics committees at each center (see Online Resource 1 for the full listing of centers); all patients (or their legal representatives) provided written informed consent before enrollment. This trial is registered at ClinicalTrials.gov, number NCT01594749.
Study treatments
Randomization and blinding of study treatments have been previously described [7]. Briefly, subjects were randomly assigned 1:1 to receive a single 150-mg dose of IV fosaprepitant (in the fosaprepitant regimen) or IV placebo (0.9% normal saline in the control regimen) approximately 30 min before MEC initiation on day 1. The combination of oral ondansetron and oral dexamethasone was administered in accordance with national and international antiemetic guideline recommendations [3, 4, 8]. In each treatment arm, both ondansetron and dexamethasone were taken before MEC initiation on day 1, followed by ondansetron 8 h after the first dose. In addition, ondansetron was administered every 12 h on days 2 and 3 in the control group, while the fosaprepitant group received matching placebo capsules. All study medications were supplied and administered in a blinded manner.
Study outcomes
As previously reported in the primary analysis, the primary efficacy end point was the proportion of subjects achieving CR during the delayed phase, and the secondary efficacy end points were the proportions of subjects achieving CR during the acute and overall phases [7]. This exploratory analysis evaluated the impact that MEC heterogeneity may have on the achievement of CR after treatment using a single-day IV fosaprepitant regimen. The main objective of this analysis was to explore the impact of chemotherapy type (carboplatin-based, non-carboplatin-based regimens) or chemotherapy duration (single-day, multiple-day) on CR achievement. Other factors influencing CR were also explored, including baseline characteristics that are known to be patient-related risk factors for CINV (sex, age, history of motion sickness, nausea in pregnancy, alcohol use).
Safety was assessed by measuring clinical adverse events (AEs) per National Cancer Institute Common Toxicity Criteria, version 4.0. The current analysis also evaluated treatment-emergent AEs (TEAEs) by chemotherapy type (carboplatin-based, non-carboplatin-based regimens).
Statistical analysis
Detailed statistical methods for the primary analysis of this study were reported previously [7]. Treatment comparisons for efficacy analyses included formal tests for superiority using the Cochran-Mantel-Haenszel test (stratified by sex); p ≤ 0.05 (two-tailed test) indicated a significant difference.
Chemotherapy type comparisons for CR were tested by odds ratios using the Cochran-Mantel-Haenszel method to adjust for treatment effect. Logistic regression analysis was performed to identify factors driving CR. Time to first emetic episode during the overall phase was assessed by determining product-limit survival estimates, and a log-rank test was used for testing group comparisons. Statistical analysis of treatment effect within the carboplatin-based and non-carboplatin-based subgroups and the single- and multiple-day therapy subgroups are presented with 95% confidence intervals by z statistics.
Effectiveness was assessed in the intention-to-treat population (ITT; subjects receiving ≥ 1 dose of study drug and analyzed according to their randomly assigned group, regardless of actual drug received). Safety was evaluated in the all-subjects-as-treated population (aSaT; subjects receiving ≥ 1 dose of study drug and analyzed based on the drug received). Descriptive statistics were used to summarize demographic variables, baseline characteristics, and AEs.
Results
Subjects
Overall, 1150 subjects were screened and 1015 were randomly assigned to either the fosaprepitant regimen (n = 508) or the control regimen (n = 507) between October 30, 2012 and November 3, 2014. Reasons for exclusion after screening included screen failure (n = 116), technical problems (n = 9), withdrawal of consent (n = 5), subject withdrawal (n = 2), physician decision (n = 1), protocol violation (n = 1), and death (n = 1). The ITT and aSaT populations consisted of 1000 and 1001 subjects, respectively. A CONSORT diagram for both the ITT and aSaT populations has been published previously [7]. Table 1 summarizes demographics and baseline characteristics for the ITT population. No notable differences were seen between groups.
Chemotherapy regimens
Chemotherapy distribution (based on data for the first dose of MEC or other non-MEC agents) was equally distributed between carboplatin-based (513/1000) and non-carboplatin-based (487/1000) regimens (Online Resource 2). Non-carboplatin agents included oxaliplatin (23.9%), cyclophosphamide (11.5%), doxorubicin (5.5%), irinotecan (2.3%), epirubicin (2.0%), bendamustine (0.6%), ifosfamide (0.2%), and other (2.7%). Single-day chemotherapy regimens accounted for 70.6% of the total ITT population (706/1000, Table 1); most of these single-day regimens were carboplatin-based (67.6%). Conversely, few subjects (13.6%) received carboplatin-based therapies as part of their multiple-day regimens. Fosaprepitant and control regimens were well balanced in the carboplatin-based and non-carboplatin-based subgroups for subjects receiving single-day and multiple-day MEC regimens (Online Resource 3).
Effect of MEC heterogeneity on CR
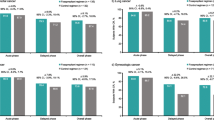
The fosaprepitant regimen during the delayed and overall phases was associated with a consistent CR rate in carboplatin-based (78.2 and 77.8%, respectively) and non-carboplatin-based (79.6 and 76.3%, respectively) subgroups, with no differences in treatment effects (1.4% delayed phase, 1.5% overall phase) (Fig. 1). Larger treatment effects favoring the fosaprepitant regimen versus control were observed for the carboplatin-based compared with the non-carboplatin-based subgroup during the delayed (14.1 vs 6.5%) and overall (14.5 vs 5.6%) phases; however, adjustment for treatment effect revealed no significant differences between the carboplatin-based and non-carboplatin-based subgroups (p = 0.0597 delayed and p = 0.3006 overall phases). For the control group, CR rates were slightly higher with non-carboplatin-based versus carboplatin-based treatment in the delayed and overall phases (treatment effect difference: 9 and 7.4%, respectively). During the acute phase, CR was achieved by most recipients in the fosaprepitant and control regimens in both the carboplatin-based (94.6 and 92.2%, respectively) and non-carboplatin-based (91.8 and 89.7%, respectively) subgroups (Fig. 1); no treatment effect differences were observed between subgroups.
The fosaprepitant regimen during the delayed and overall phases was associated with a consistent CR rate whether duration of chemotherapy was single day (77.9 and 76.3%, respectively) or multiple day (80.3 and 78.0%, respectively), with no difference in treatment effects (differences: 2.4% delayed phase, 1.7% overall) (Fig. 2). Larger treatment effects favoring the fosaprepitant regimen versus control were observed for the single-day versus the multiple-day subgroup during the delayed (13.2 vs 3.2%) and overall (12.8 vs 4.0%) phases. After adjustment for treatment effect, a significant difference between the single-day and multiple-day subgroups was observed during the delayed phase (p = 0.0199), but not the overall phase (p = 0.0596). For the control regimen, CR was slightly higher for the multiple-day versus the single-day MEC subgroup (treatment effect differences: 12.4% delayed phase, 10.5% overall). Most recipients (> 89%) experienced CR during the acute phase in both subgroups (Fig. 2); no treatment effect differences were seen between subgroups. Analysis by tumor type revealed that most subjects receiving multiple-day carboplatin-based chemotherapy had lung cancer (32/35 subjects); all subjects in this subgroup received etoposide, and all but one experienced CR (31/32 subjects with lung cancer) (Online Resource 4).
The fosaprepitant regimen significantly delayed onset of a first emetic episode during the overall phase compared with the control regimen in the carboplatin-based and the non-carboplatin-based subgroups (p < 0.0001) (Fig. 3a). The fosaprepitant regimen also significantly delayed onset of a first emetic episode compared with the control regimen in the single-day and the multiple-day subgroups (p < 0.0001) (Fig. 3b).
Most subjects in the fosaprepitant arm experienced CR during the delayed phase with carboplatin-based and non-carboplatin-based MEC agents, whether they were single- or multiple-day regimens. In subjects receiving single-day regimens, CR was experienced in the fosaprepitant and control arms, respectively, by 76.5% (182/238) and 61.5% (147/239) of subjects receiving carboplatin-based regimens, 69.8% (37/53) and 78.6% (44/56) of subjects receiving cyclophosphamide-based regimens, 88.9% (24/27) and 64.3% (18/28) of subjects receiving doxorubicin-based regimens, 64.3% (9/14) and 83.3% (5/6) of subjects receiving epirubicin-based regimens, and 80.0% (20/25) and 37.5% (6/16) receiving oxaliplatin-based chemotherapy (Online Resource 5). In subjects receiving multiple-day chemotherapy regimens, CR was experienced in the fosaprepitant and control arms, respectively, by 94.7% (18/19) and 93.8% (15/16) of subjects receiving carboplatin-based chemotherapy, by 80.0% (8/10) and 75.0% (9/12) of subjects receiving irinotecan-based chemotherapy, and 73.7% (70/95) and 71.3% (72/101) receiving oxaliplatin-based chemotherapy (Online Resource 6).
When all factors that could influence CR were evaluated simultaneously in logistic regression analyses (baseline characteristics and study treatments), no statistically significant difference was seen between carboplatin-based and non-carboplatin-based therapies. Three prognostic factors were considered significant drivers of CR: treatment effect (fosaprepitant vs control; p = 0.0002), sex (females at higher risk; p = 0.0001), and interaction of chemotherapy type (carboplatin) and chemotherapy duration (multiple day) (p = 0.0357).
Effect of MEC heterogeneity on safety
The fosaprepitant regimen was well tolerated. In the primary safety analysis (aSaT population), the proportion of subjects who experienced ≥ 1 AE was similar between the fosaprepitant and control groups (61.9 vs 60.8%) [7]. When evaluating AEs by chemotherapy type (Table 2), the overall incidence of AEs was similar in subjects who received carboplatin-based compared with non-carboplatin-based therapies, trending toward fewer TEAEs in the carboplatin-based subgroup (57.7 vs 65.3%; difference − 7.6%). The most frequently reported TEAEs (occurring in ≥ 10% in either group) were fatigue, diarrhea, and constipation (Table 2).
Discussion
The findings of this exploratory analysis showed consistent efficacy of the fosaprepitant regimen across all phases (acute, delayed, overall), irrespective of chemotherapy type (carboplatin-based, non-carboplatin-based regimens) and duration (single-day, multiple-day). The analysis also showed that almost an equal number of subjects received carboplatin-based therapies (CINV risk at the upper end of the MEC spectrum) and non-carboplatin-based MEC therapies. This highlights the heterogeneity of a “true” MEC population, which consists of MEC agents on a broad CINV risk spectrum.
Although treatment effect of the fosaprepitant regimen seemed more pronounced in the carboplatin-based subgroup than the non-carboplatin-based subgroup, this difference was statistically nonsignificant when adjusted for treatment. As expected, in the delayed and overall phases, the control group displayed greater CR in the non-carboplatin-based subgroup (73.1 and 70.7%, respectively) versus the carboplatin-based subgroup (64.1 and 63.3%); subjects receiving fosaprepitant exhibited higher but similar CR rates in the non-carboplatin-based (79.6 and 76.3%) and carboplatin-based (78.2 and 77.8%) subgroups. Differences in fosaprepitant treatment effect between single-day and multiple-day chemotherapy regimens might be driven by the interaction between carboplatin and multiple-day therapies: a greater treatment difference was observed in the single-day regimens. However, the population in this subgroup was not heterogeneous: most (32/35) subjects had lung cancer, and all subjects with lung cancer received etoposide plus carboplatin. Although a carboplatin-based MEC regimen was used most often, other MEC agents constituted approximately 50% of the therapies. The effectiveness of the fosaprepitant regimen was excellent regarding CR for subjects who received selected non-carboplatin MEC agents, including oxaliplatin, doxorubicin, and irinotecan. These findings support the primary analysis results, indicating that the fosaprepitant regimen was effective in the overall MEC population.
The observed treatment effect for the single-day fosaprepitant regimen (relative to the standard 3-day combination of 5-HT3 and dexamethasone) in subjects receiving non-AC carboplatin-based MEC regimens resembled that of other NK-1 receptor antagonist studies. Absolute differences for CR in subjects who received carboplatin-based chemotherapy (overall phase) were 15% for fosaprepitant (current study), compared with 10–15% for aprepitant [9, 10] and 15% for rolapitant [11]. A recent post hoc analysis of a phase III trial evaluated the efficacy and safety of rolapitant in subjects who received non-AC MEC [12]. In the delayed phase, the absolute CR difference for the rolapitant regimen versus the control regimen was similar to that of the fosaprepitant regimen in the current analysis (6.7 and 6.5%, respectively; both statistically nonsignificant). Treatment effect for the rolapitant regimen was significant (12.8%; p = 0.049). However, the published rolapitant regimen included 3 days of a 5-HT3 receptor antagonist [12], whereas the current study provided a single day of an antiemetic agent prophylactically. Overall, safety findings were generally in line (i.e., similar TEAEs) with those expected in patients with cancer and were consistent with those of other trials.
The current analysis was post hoc and exploratory, and, although conclusions support primary findings [6], additional conclusions regarding MEC heterogeneity require prospective studies for confirmation. The number of patients in some subgroups was small, particularly those receiving multiple-day carboplatin-based regimens (n < 20 for fosaprepitant and control arms) compared with those receiving single-day carboplatin-based regimens (n > 200 for fosaprepitant and control arms); therefore, the findings should be interpreted carefully.
Conclusions
Results of the current exploratory analysis of this phase III trial support the primary findings that a single-day fosaprepitant regimen is effective in preventing CINV in subjects receiving non-AC MEC in single-day and multiple-day chemotherapy regimens. Chemotherapy type and duration did not appear to influence response to fosaprepitant. The fosaprepitant regimen was effective in carboplatin-based and non-carboplatin-based MEC regimens, although treatment effect seemed more pronounced in the carboplatin-based subgroup. Adequately powered, prospective studies are necessary to further define the effects of non-carboplatin-based MEC regimens on antiemetic response to NK-1 receptor antagonist.
References
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133. https://doi.org/10.1093/annonc/mdw270
Jordan K, Jahn F, Aapro M (2015) Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol 26(6):1081–1090. https://doi.org/10.1093/annonc/mdv138
National Comprehensive Cancer Network (2016) National Comprehensive Cancer Network guidelines for supportive care. Antiemesis. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 10 Nov 2017
Jordan K, Gralla R, Jahn F, Molassiotis A (2014) International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol 722:197–202. https://doi.org/10.1016/j.ejphar.2013.09.073
(2017) Emend [package insert]. Whitehouse Station, NJ; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc
(2017) Emend for injection [package insert]. Whitehouse Station, NJ; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc
Weinstein C, Jordan K, Green SA, Camacho E, Khanani S, Beckford-Brathwaite E, Vallejos W, Liang LW, Noga SJ, Rapoport BL (2016) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial. Ann Oncol 27(1):172–178. https://doi.org/10.1093/annonc/mdv482
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29(31):4189–4198. https://doi.org/10.1200/JCO.2010.34.4614
Ito Y, Karayama M, Inui N, Kuroishi S, Nakano H, Nakamura Y, Yokomura K, Toyoshima M, Shirai T, Masuda M, Yamada T, Yasuda K, Hayakawa H, Suda T, Chida K (2014) Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 84(3):259–264. https://doi.org/10.1016/j.lungcan.2014.03.017
Yahata H, Sonoda K, Kobayashi H, Shimokawa M, Ohgami I, Saito T, Ogawa S, Sakai K, Ichinoe A, Ueoka Y, Hasuo Y, Nishida M, Oishi R, Kato K (2014) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting with a moderately emetogenic chemotherapy: a multicenter, placebo-controlled, double-blind, randomized study in Japanese gynecologic patients receiving paclitaxel and carboplatin. Ann Oncol 25(suppl 4):iv518 Abstract 1481PD
Hesketh PJ, Schnadig ID, Schwartzberg LS, Modiano MR, Jordan K, Arora S, Powers D, Aapro M (2016) Efficacy of the neurokinin-1 receptor antagonist rolapitant in preventing nausea and vomiting in patients receiving carboplatin-based chemotherapy. Cancer 122(15):2418–2425. https://doi.org/10.1002/cncr.30054
Hesketh PJ, Schwartzberg LS, Modiano MR, Arora S, Poma A, Schnadig ID (2015) Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting (CINV) in moderately emetogenic therapy (MEC). J Clin Oncol 33(suppl):abstr 9622
Acknowledgements
The authors thank LiWen Liang (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for assisting with data collection and analysis. Medical writing and editorial assistance were provided by Traci Stuve and Maxwell Chang of ApotheCom, Yardley, PA, USA and funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by ethics committees at each center (see Online Resource 1 for the full listing of centers); all patients (or their legal representatives) provided written informed consent before enrollment. This trial is registered at ClinicalTrials.gov, number NCT01594749.
Conflict of interest
Dr. Weinstein is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and is a stockholder in the company. Dr. Jordan received consultant fees from Merck Sharp & Dohme Corp., Helsinn, and Tesaro. Dr. Green is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and is a stockholder in the company. Dr. Camacho has nothing to disclose. Dr. Khanani has nothing to disclose. Dr. Beckford-Brathwaite is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and is a stockholder in the company. Dr. Pong is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and is a stockholder in the company. Dr. Noga is now an employee of Takeda Oncology. Dr. Rapoport acted on advisory boards for Merck & Co., Inc., Tesaro, and Heron Therapeutics, and acted on speakers’ bureaus for Tesaro.
Electronic supplementary material
Online Resource 1
(PDF 402 kb)
Online Resource 2
MEC chemotherapeutic regimens received by subjects in study PN031 (JPG 2591 kb)
Online Resource 3
Chemotherapeutic categories received by chemotherapy type and duration. aProtocol deviations, including regimens with low emetogenic chemotherapy only, highly emetogenic chemotherapy, and chemotherapy on day 2 only. Carbo carboplatin, MEC moderately emetogenic chemotherapy (JPG 82 kb)
Online Resource 4
(PDF 89 kb)
Online Resource 5
(PDF 93 kb)
Online Resource 6
(PDF 165 kb)
Rights and permissions
About this article
Cite this article
Weinstein, C., Jordan, K., Green, S.A. et al. Evaluation of factors contributing to the response to fosaprepitant in a heterogeneous, moderately emetogenic chemotherapy population: an exploratory analysis of a randomized phase III trial. Support Care Cancer 26, 3773–3780 (2018). https://doi.org/10.1007/s00520-018-4242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4242-x