Abstract
Exercise is recommended following cancer diagnosis and may be particularly valuable for women receiving cardiotoxic chemotherapy treatments. We investigated breast cancer patient preference on exercise programming in a prospective manner and retrospectively assessed length of time between diagnosis and chemotherapy initiation. Sixty-seven newly diagnosed breast cancer patients responded to questions regarding exercise programming related to cancer treatment and surveys on current activity level. Additionally, a retrospective chart review was conducted on 500 random breast cancer patients. Age, cancer stage, treatment, and treatment dates were extracted. Women were interested in, or, absolutely wanted to, participate in an exercise program before treatment (76.2%). There was uncertainty regarding willingness to delay treatment; 49.2% were willing to delay their treatment if the program was recommended by their doctors, 41.8% would not, and 9.0% were too unsure to respond. However, women would like to hear information about an exercise program for cancer patients when they are first diagnosed (61.9%). We observed that 64.6% of women were below recommended levels of physical activity; yet, current activity was not associated with an interest in an exercise program or willingness to delay treatment. Retrospectively, we observed an average interval of 72.6 ± 34.6 days between cancer diagnosis and initiation of anthracycline-based chemotherapy treatment, with younger women with more advanced cancer receiving anthracycline-based chemotherapy. Based on patient preference and length of time to chemotherapy initiation, a reasonable next step to promote the current recommendations for exercise could be to integrate exercise into breast cancer care earlier in treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several national and international agencies recommend exercise participation for all persons following a cancer diagnosis [1,2,3]. The current evidence suggests that aerobic exercise training is safe during neoadjuvant and adjuvant therapy [4,5,6,7,8]. Importantly, this evidence also indicates that aerobic exercise training programs (50–75% of baseline exercise capacity for 12–15 weeks) improve cardiopulmonary function and patient-related outcomes [1, 9].
Increases of 13.3% in cardiopulmonary fitness have been observed with aerobic exercise training programs during neoadjuvant chemotherapy [7]. Cardiopulmonary fitness is highly predictive of overall and cardiovascular specific mortality in women [10, 11]. Specifically, an increase in cardiopulmonary fitness of approximately 10% has been associated with a 19% reduction in risk for cardiovascular mortality [12]. This is important as breast cancer patients already present at diagnosis with 31% lower cardiopulmonary fitness levels compared to healthy age-matched women [13].
This enhanced risk for cardiovascular mortality in breast cancer patients is further compounded by cardiotoxic anthracycline containing chemotherapy, which causes permanent cardiac damage through a variety of mechanisms [14,15,16]. Cumulative doses of doxorubicin well below the treatment ceiling of 550 mg/m2 have been shown to induce subclinical impairment of cardiac function [17,18,19]. Long-term consequences of subclinical left ventricular dysfunction seen in cardiotoxicity are not entirely known. However, they appear to increase the susceptibility to progressive cardiac dysfunction associated with aging and cardiovascular disease (CVD) as estimates indicate that breast cancer survivors have an increased risk of heart failure and coronary artery disease (hazard ratio: 1.95 and 1.27, respectively) [20].
Pre-clinical studies of exercise training prior to chemotherapy have reported a significant attenuation of anthracycline-induced cardiac injury by a variety of mechanisms [21,22,23,24,25]. However, no study to date has investigated the preventative effects of an exercise intervention before chemotherapy on subclinical changes in cardiac function or biomarkers of cardiac damage. The reluctance to initiate an intervention at this time point may be due to the perceived urgency to initiate curative treatment, or, unfamiliarity with the benefits of exercise. Yet, given the clinical recommendations for exercise following a cancer diagnosis, and recent work investigating exercise training during neoadjuvant chemotherapy, we were interested in the feasibility of implementing exercise programming earlier in the breast cancer care continuum for women not receiving neoadjuvant chemotherapy. We sought to characterize patient preference regarding such an intervention given the burden women already face with their cancer care and also determine the average window of opportunity between diagnosis and chemotherapy initiation. In order to do this, we used two aims: (1) we surveyed a group of newly diagnosed breast cancer patients on their preferences regarding exercise in their cancer care and (2) we conducted a medical record review on a larger sample to distill average time between diagnosis and chemotherapy initiation. The goal of this study was to use a multi-modal assessment of important feasibility issues in exercise programming for clinical breast cancer care.
Methods
Setting
The study was conducted at the University of Pennsylvania Hospital and Pennsylvania Hospital and was approved by the institutional review board of the University of Pennsylvania. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Eligibility criteria for the questionnaire portion of the study (aim 1) included being English-speaking, female, between the ages of 18 and 80, and had been diagnosed with breast cancer. From 2012 to 2014, women were recruited at a pre-surgical appointment with a breast surgeon where informed consent was obtained. Participants completed an interviewer administered questionnaire and self-reported on leisure time activity as well as behavioral stage of change.
Procedure and measures
The questionnaire consisted of four multiple choice questions. Women were asked if they would be interested in participating in an exercise program before chemotherapy initiation (should chemotherapy be recommended), if they would be willing to put off their chemotherapy treatment (should chemotherapy be recommended), who they would like to hear information about exercise programming from, and when they would like to know information about exercise programming in relationship to their cancer treatment. Additionally, the Godin Leisure-Time Exercise Questionnaire and the Exercise Stages of Change-short form were administered [26, 27]. Leisure Score Index (LSI) was calculated from the Godin Leisure-Time Exercise Questionnaire. Scores 24 and higher are associated with activity at or above the public health recommendation of 150 min/week of moderate physical activity [28].
A separate retrospective chart review (aim 2) of 500 women treated for breast cancer at the University of Pennsylvania in 2011 was conducted. Women were randomly selected via computer-generated randomization methods. We determined the time interval between breast cancer diagnosis (defined as the date of the diagnostic breast biopsy procedure) and chemotherapy initiation. Clinical information, including age, ethnicity, diagnosis date, cancer stage, summary of first course of treatment, starting date of chemotherapy, timing of chemotherapy (neoadjuvant or adjuvant), and drugs used during chemotherapy, was obtained from a review of electronic medical records by an independent researcher (KS).
Statistical analysis
χ2 was used to test independence of distribution of categorical data for each survey question. ANOVA was used to assess differences between continuous variables for each survey question. Survey responses to questions 2 and 3 were collapsed into more robust categories for analysis. Students’ t test and χ2 were used to assess differences between women treated with or without anthracycline chemotherapy. P values < 0.05 were considered to indicate statistical significance. All testing and descriptive statistics were carried out using STATA software version 12.0.
Results
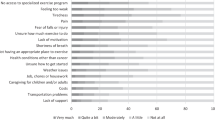
Sixty-seven women, aged 55.3 ± 11.6 years, completed the questionnaire portion of the study (Table 1). Our response rate was 69.6%. On average, patients were Caucasian (71.6%), overweight (BMI: 28.4 ± 6.1 kg/m2), parous, and post-menopausal. Lumpectomy was the predominant surgical recommendation (71.6%) and the majority of breast cancer patients had stage 1 cancer (61.2%) which was hormone sensitive (75.8% ER/PR+). The study questions posed and responses recorded are displayed in Table 2. Most women absolutely wanted to or were at least interested in participating in an exercise program before treatment (76.2%). As exercise initiated before cardiotoxic chemotherapy and radiation may be beneficial, we asked women if they would be willing to delay their treatment in order to implement an exercise program as part of their clinical care. Forty-nine percent of the study cohort was amenable to delaying treatment, with the important caveat that they would only put off treatment if their physician recommended doing so. A physician may not need to be the gate keeper to deliver detailed exercise programming information as 58.5% of participants would be open to hearing such information from a cancer exercise specialist. Lastly, the majority of women were interested in receiving the information about exercise programming in their cancer care when they were diagnosed (61.9%), compared to when they were first told about an abnormal screening (15.9%), or compared to hearing this information yearly from their gynecologist (22.2%).
Similar to current trends in the US population for physical activity, 64.6% of the study population was sedentary, as only 35.4% were at or above the public health recommendation for 150 min per week of moderate to vigorous physical activity (Table 3). However, 50.0% of the study population believed that they were exercising regularly (Table 3). A small percentage (9.1%) did not intend to start regular exercise at all.
We next investigated if demographics, current physical activity levels, or behavioral stage of change were associated with responses to exercise programming. Responses to interest in exercise programming, delaying treatment to initiate exercise programming, and who should deliver information on exercise programming were not different by BMI, ethnicity, or presence of comorbidities (Table 4). There was a trend (P = 0.07) for younger women to desire information on exercise programming prior to their breast cancer diagnosis. There was also a trend (P = 0.07) for women whom exercised at or above public health recommendations for physical activity to be more willing to delay breast cancer treatment for exercise programming, compared to women that did not meet public health recommendations for physical activity. We did not see any differences in responses to the questionnaire by self-reported behavioral change.
Given patient interest in exercise pre-habilitation, and the preference for this modality to be introduced at cancer diagnosis, we investigated the length of the treatment interval between diagnosis of breast cancer and initiation of anthracycline containing chemotherapy treatment. After chart review of 500 patients diagnosed with breast cancer and treated at the HUP, we noted the number of breast cancer patients that (1) received chemotherapy (179 women, 35.8% of breast cancer patients) and (2) received anthracycline containing chemotherapy (83 women, 16.6% of breast cancer patients, 46.4% of breast cancer patients receiving chemotherapy) (Fig. 1a). Further, 11 women received neoadjuvant anthracycline containing chemotherapy, while 72 women received adjuvant anthracycline containing chemotherapy (72 women, 14.4% of breast cancer patients, 40.2% of breast cancer patients receiving chemotherapy, and 86.7% of breast cancer patients receiving anthracycline containing chemotherapy). On average, we observed 72.6 ± 34.6 days (median 68 days, range 17–164 days) between cancer diagnosis and initiation of anthracycline based adjuvant chemotherapy treatment (Fig. 1b).
A retrospective chart review of randomly selected breast cancer cases was conducted (a). We assessed the number of patients who received anthracycline containing chemotherapy regimens as part of their clinical care. We also assessed the length of time between the date of diagnostic breast biopsy and the date of initiation of anthracycline-based adjuvant chemotherapy treatment (b)
Demographic data on the 83 women that received anthracycline containing chemotherapy and the 96 women receiving non-anthracycline containing chemotherapy are presented in Table 5. Women treated with anthracyclines were significantly younger and presented with significantly more advanced cancer stage compared to women receiving non-anthracycline containing chemotherapy.
Discussion
To better understand feasibility of exercise programming in clinical breast cancer care, we investigated patient interest, preference on timing and delivery, relationships between patient preference and patient characteristics, as well as temporal clinical progression of treatment. Our findings are preliminary, but suggest that breast cancer patients are interested in the clinical use of exercise programming, and that patients are open to cancer exercise specialists delivering information regarding the programming. Further, if the patient’s physician is in agreement that initiating lifestyle modifications will aid in clinical breast cancer care, patients may be more willing to delay their curative treatment at the physician’s recommendation. Additionally, while our cohort was predominantly sedentary, we observed that participants who were more physically active were more likely to be open to delaying their treatment. However, delaying treatment may not be necessary. We observed that women receiving adjuvant anthracycline containing chemotherapy had an average of 72.6 days between diagnosis and start of chemotherapy.
In this study, we observed a high interest level in exercise programming which was similar to other studies [29]. Additionally, we did not observe any patient characteristics which identified participants that were not interested in exercise programming. Neither current physical activity level nor presence of comorbidities was associated with disinterest or uncertainty regarding exercise programming. Others have also reported strong unbiased initial interest in exercise programming which may be due to the “teachable moment” seen following cancer diagnosis [30, 31].
Retrospective reports indicate that breast cancer patients are highly motivated to make lifestyle changes, specifically during the period following diagnosis and before treatment. Jones et al. showed that 38.8% of breast cancer survivors reported they would have preferred receiving exercise counseling before treatment, compared to 18.7% during treatment, 21.5% immediately following treatment, and 21.2% 3 months or more following treatment [32]. Our prospective study confirms patient interest in exercise counseling before treatment [33, 34]. Not only are patients interested in an exercise program before treatment, but breast cancer patients have specifically identified a preference for exercise counseling at the point of diagnosis. This selection was in comparison to options of exercise counseling prior to a confirmed diagnosis (yearly at gynecologist, or at time of abnormal screening result). It must be noted though that patient preference for exercise programming and intentions may not correlate well with behavior. Indeed, Broderick et al. report that from 6 weeks following completion of chemotherapy through 1 year following completion of chemotherapy, breast cancer survivors showed no differences in physical activity level compared to a comparison (non-cancer) group [35].
During chemotherapy, support for behavior change and particularly with regard to exercise-oncology will likely be necessary to observe clinically meaningful responses. We report that breast cancer patients are open to cancer exercise specialists delivering exercise interventions. A total of 47.7% of women in this study reported that they would prefer to receive exercise counseling from a cancer exercise specialist as opposed to a physician, and 10.8% are open to hearing this information from either a cancer exercise specialist or a physician. This is similar to young-adult cancer survivors (49.6%), while a higher number (77%) of older-adult cancer survivors would prefer to receive exercise counseling from an exercise specialist affiliated with a cancer center [29, 32].
Having cancer exercise specialists deliver exercise programming early in breast cancer treatment would be advantageous as we observed a window of opportunity that would be clinically meaningful. We observed an average interval of 72 days between breast cancer diagnosis and star of adjuvant anthracycline containing chemotherapy. We explored this interval in breast cancer care specifically for women who received anthracycline containing chemotherapy because of known cardiotoxicity associated with anthracyclines. Pre-clinical studies have indicated that both chronic and acute exercise training prior to doxorubicin administration is protective against anthracycline-induced cardiac damage [22,23,24,25].
No clinical trial has been done to investigate whether exercise prior to chemotherapy initiation is effective in preventing or mitigating cardiotoxicity in humans. However, studies have shown that as little as 4 weeks of exercise training can significantly improve cardiac and vascular function in patients with comorbidities such as obesity [36], hypertension [37], and coronary artery disease [38]. Not only can exercise significantly impact cardiovascular structure and function in a short amount of time, but cellular adaptations such as increasing antioxidant expression [39], mitochondrial energy metabolism [40], and activity of DNA repair enzymes [41, 42] are important in mitigating cardiotoxicity.
Cardiotoxic chemotherapy is part of the “multi-hit” hypothesis by Jones et al. [43]. This hypothesis posits that breast cancer patients are already at risk for CVD, are exposed to treatment induced damages, and will continue to be subjected to cardiovascular insults due to lifestyle and aging [43]. In our study, 43% of women who completed the questionnaire had at least one comorbidity at diagnosis and thus are already at risk, if not already presenting with CVD. Also in our study, women who received anthracycline containing chemotherapy were significantly younger than women who received non-anthracycline containing chemotherapy. As women live longer following a breast cancer diagnosis, they increase their risk for dying from other causes of death, of which CVD is the leading cause [44]. Thus, as women receiving anthracycline containing chemotherapy tend to be younger, there may be even greater long-term risk for CVD in this population.
An important finding of our study is that only 14.4% of women in this study received adjuvant anthracycline containing chemotherapy. Further, while the observed window of opportunity for exercise programming was 72.6 days between diagnosis and start of chemotherapy, how this interval works clinically needs to be acknowledged. Pathology results and a formal cancer diagnosis are often followed by an initial clinic visit with a breast surgeon. At this point, a breast cancer patient’s oncological treatment plan is not formalized. Thus, identifying breast cancer patients during their first clinic visit that will be receiving anthracycline containing chemotherapy is not possible. However, waiting to initiate exercise programming until patients are recommended for anthracycline containing chemotherapy will significantly reduce the amount of time available for physiological adaptation to exercise.
Exercise, and avoidance of inactivity, is clinically recommended for all cancer patients [1,2,3]. Therefore, exercise programming can be initiated for all, as clinically recommended, and further tailored for those at the highest risk for cardiotoxicity. Specifically, upon positive biopsy results, a cancer exercise specialist could contact the patient via phone and introduce information regarding increasing physical activity level. Then, a cancer exercise specialist embedded in the cancer center could see newly diagnosed patients immediately following a patient’s clinic visit with the breast surgeon. Such an approach would limit patient burden while increasing comprehensive care for all women.
A limitation of our study is the small sample size associated with the interviewer administered questionnaire. The sample also was drawn from an urban academic medical center. Generalizability for rural populations or other cancer institutes may be difficult due to differences in perceptions of health and wellness. Additionally, as the questionnaire was interviewer administered, there is likely reporting bias in the form of social desirability. This may affect responses towards more positive health beliefs/behaviors, such as participant interest in exercise programming or desire to hear about exercise programming from a cancer exercise specialist. Further, this study did not assess objective measures of physical activity and relied on self-report. Thus, there is high probability for under reporting the number of sedentary individuals [45]. Specific to this study, we observed a trend for women that self-reported higher physical activity levels to be more open to delaying their treatment compared to women that self-reported lower physical activity levels. This trend would likely not remain if we had objective measures of physical activity levels, and this conclusion is supported by the observed lack of association between stage of change and patient choice in treatment delay/timing. While this study provides some insight on the acceptability of hypothetical exercise programming in breast cancer treatment, future research will require a mixed method approach (qualitative and quantitative) to examine the acceptability of a pilot exercise program in clinic. Future research with a pilot exercise program in clinic will provide critical observations for dissemination and implementation.
In summary, this study provides preliminary observations on patient preference with regard to exercise programming in clinical breast cancer care. We show that while the differential value of exercise in relationship to treatment timing is currently unknown, there is a window of opportunity within current clinical care to potentially implement an intervention for patients receiving chemotherapy. Together with supportive data from preclinical studies on exercise-induced cardio-protection against anthracycline-induced damage, and clinical studies on exercise during chemotherapy, this study highlights the plausibility of initiating these supportive interventions as early as possible in breast cancer care.
References
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426
Hayes SC, Spence RR, Galvao DA, Newton RU (2009) Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport 12(4):428–434
van den Berg JP, Velthuis MJ, Gijsen BC, Lindeman E, van der Pol MA, Hillen HF (2011) guideline “cancer rehabilitation”. Ned Tijdschr Geneeskd 155(51):A4104
Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, Snyder DC, Giguere JK, Shaw E (2008) Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer 8(1):70–79
Vincent F, Labourey JL, Leobon S, Antonini MT, Lavau-Denes S, Tubiana-Mathieu N (2013) Effects of a home-based walking training program on cardiorespiratory fitness in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Eur J Phys Rehabil Med 49(3):319–329
Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R (2001) Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol 19(3):657–665
Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW (2014) Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol 53(1):65–74
Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C, Dolan LB, Forbes CC, Wooding E, Trinh L, Segal RJ (2013) Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst 105(23):1821–1832
Sturgeon KM, Ky B, Libonati JR, Schmitz KH (2014) The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat 143(2):219–226
Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW (1996) Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276(3):205–210
Vigen R, Ayers C, Willis B, DeFina L, Berry JD (2012) Association of cardiorespiratory fitness with total, cardiovascular, and noncardiovascular mortality across 3 decades of follow-up in men and women. Circ Cardiovasc Qual Outcomes 5(3):358–364
Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW 3rd, Blair SN (2011) Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the aerobics center longitudinal study. Circulation 124(23):2483–2490
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, Haykowsky M (2012) Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30(20):2530–2537
Bovelli D, Plataniotis G, Roila F (2010) Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol 21(Suppl 5):v277–v282
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET (2012) Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18(11):1639–1642
Chung WB, Yi JE, Jin JY, Choi YS, Park CS, Park WC, Song BJ, Youn HJ (2013) Early cardiac function monitoring for detection of subclinical doxorubicin cardiotoxicity in young adult patients with breast cancer. J Breast Cancer 16(2):178–183
Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23(31):7811–7819
Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, Dumontet C, Thieblemont C, Arnaud P, Antal D, Bouafia F, Coiffier B (2004) Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol 22(10):1864–1871
Khan NF, Mant D, Carpenter L, Forman D, Rose PW (2011) Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer 105(Suppl 1):S29–S37
Ascensao A, Magalhaes J, Soares J, Ferreira R, Neuparth M, Marques F, Oliveira J, Duarte J (2005) Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol 100(3):451–460
Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA (2005) Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Phys Heart Circ Phys 289(2):H722–H731
Kavazis AN, Smuder AJ, Min K, Tumer N, Powers SK (2010) Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol 299(5):H1515–H1524
Chicco AJ, Schneider CM, Hayward R (2005) Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol 289(2):R424–R431
Wonders KY, Hydock DS, Schneider CM, Hayward R (2008) Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther 7(3):147–154
Prochaska JO, DiClemente CC (1983) Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 51(3):390–395
Godin G, Shephard RJ (1985) A simple method to assess exercise behavior in the community. Can J Appl Sport Sci Journal canadien des sciences appliquees au sport 10(3):141–146
Amireault S, Godin G, Lacombe J, Sabiston CM (2015) The use of the Godin-Shephard leisure-time physical activity questionnaire in oncology research: a systematic review. BMC Med Res Methodol 15:60
Belanger LJ, Plotnikoff RC, Clark A, Courneya KS (2012) A survey of physical activity programming and counseling preferences in young-adult cancer survivors. Cancer Nurs 35(1):48–54
Lawson PJ, Flocke SA (2009) Teachable moments for health behavior change: a concept analysis. Patient Educ Couns 76(1):25–30
Vassbakk-Brovold K, Berntsen S, Fegran L, Lian H, Mjaland O, Mjaland S, Seiler S, Kersten C (2015) Individualized comprehensive lifestyle intervention in patients undergoing chemotherapy with curative or palliative intent: who participates? PLoS One 10(7):e0131355
Jones LW, Courneya KS (2002) Exercise counseling and programming preferences of cancer survivors. Cancer Pract 10(4):208–215
Henriksson A, Arving C, Johansson B, Igelstrom H, Nordin K (2016) Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns 99(7):1220–1226
Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E (2000) Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 88(3):674–684
Broderick JM, Hussey J, Kennedy MJ, O'Donnell DM (2014) Testing the 'teachable moment' premise: does physical activity increase in the early survivorship phase? Support Care Cancer : Off J Multinational Assoc Support Care Cancer 22(4):989–997
Millen AM, Norton GR, Avidon I, Woodiwiss AJ (2013) Effects of short-term exercise-training on aortic systolic pressure augmentation in overweight and obese individuals. Eur J Appl Physiol 113(7):1793–1803
Montero D, Roche E, Martinez-Rodriguez A (2014) The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 173(3):361–368
Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G (2000) Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342(7):454–460
Gomez-Cabrera MC, Domenech E, Vina J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44(2):126–131
Ren J, Pulakat L, Whaley-Connell A, Sowers JR (2010) Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 88(10):993–1001
Radak Z, Chung HY, Koltai E, Taylor AW, Goto S (2008) Exercise, oxidative stress and hormesis. Ageing Res Rev 7(1):34–42
Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S (2002) Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch 445(2):273–278
Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR (2007) Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 50(15):1435–1441
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD (2011) Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13(3):R64
Mazzoni AS, Nordin K, Berntsen S, Demmelmaier I, Igelstrom H (2017) Comparison between logbook-reported and objectively-assessed physical activity and sedentary time in breast cancer patients: an agreement study. BMC Sports Sci Med Rehabil 9:8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted at the University of Pennsylvania Hospital and Pennsylvania Hospital and was approved by the institutional review board of the University of Pennsylvania. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
ᅟ
Conflicts of interest
Kathleen Sturgeon: none; Carla Fisher: none, Gina McShea: none, Susan Kruse Sullivan: none, Dahlia Sataloff: has given expert testimony to MedicoLegal, received honoraria and payment for development of educational presentations from Chest Prep (online course), and Kathryn Schmitz: received U54CA155850 from NCI. All authors have full control of primary data and agree to allow the journal to review data as requested.
Rights and permissions
About this article
Cite this article
Sturgeon, K.M., Fisher, C., McShea, G. et al. Patient preference and timing for exercise in breast cancer care. Support Care Cancer 26, 507–514 (2018). https://doi.org/10.1007/s00520-017-3856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3856-8