Abstract
Purpose
Body image is a psychological dimension of the experience of cancer, which varies along the clinical features of the disease itself and in its phases, as well as its effects in terms of functioning and quality of life. In 2012, Supportive Care in Cancer published a review addressing the relevance, application, and instruments of body image assessment for oncological settings. Since then, many research papers have been published on this topic and new questionnaires for assessing body image in oncology are now available. This contribution aims to offer both researchers and clinicians an updated review of body image assessment tools.
Methods
We searched PubMed, Psychology and Behavioral Sciences Collection, and Scopus databases, which allowed us to identify pertinent papers, classified according to the body image tool to which they refer. We then extracted the characteristics and the psychometric properties from each study.
Results
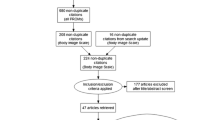
From the 657 initial records, 23 papers met the selection criteria referring to 8 body image measurements. Although increasing in number and being the subject of a growing number of studies, these papers are still not exhaustive with respect to the verified psychometric properties. In particular, it is worth noting that their applicability to all types of cancer is limited and that a focus on women with breast cancer prevails.
Conclusion
A complete validation (including a study of all types of validity and reliability) and an indication of the case results are not currently available for any of the eight instruments described. However, studies designed to apply body image assessment tools to patients other than those experiencing breast cancer as well to cultural contexts other than English-speaking countries, are increasing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The body determines the space we occupy in the world, and mediates our interactions with the physical and social world. In addition, people interact with us, approaching our corporeality.
Cancer and anti-cancer treatments affect the body in various ways, directly or in the form of side effects [1–5]. In oncology, the physical damage may be temporary or permanent, sudden or gradual, and visible or not visible (e.g. regarding internal organs). It may include functional implications or disability. It may affect organs that have an important symbolic value and/or are connected, more than others, to personal identity (reproductive organs, for example). Sometimes, the damage may be for prophylactic reasons, as in the case of preventive bilateral mastectomy in the presence of mutations on the BRCA1/2 genes.
Body image is a psychological construct that captures the perceptions, emotions, and attitudes a person holds towards his/her own body [6, 7]. In oncology, body image relates to the subjective experience of having cancer, which varies according to the clinical features of the disease itself and its phases as well as the effects in terms of functioning and quality of life.
The first use of body image assessment was related to eating disorders, for which the perception of body size and its appreciation are the most important dimensions [6, 7]. In oncology, the assessment of body image is complicated because it must take into account the different objective impairments of the body caused by the disease and/or by treatment (amputation, scarring, functional damage, infertility, alopecia, edema, etc.); their possible variations over time; the possible functional implications, as well as the esthetics; and their subjective, social, and cultural significance [1–3].
This paper aims to review instruments that were specifically designed or adapted to investigate body image in cancer patients, describing their characteristics and their psychometric properties. It represents an update of a previous preliminary review [1], and it was stimulated by the increasing numbers of papers dealing with body image and body image assessment in oncology that have been published since that work.
Method
Data sources and search
A computer-based literature search was performed to identify articles about tools used in assessing body image in cancer patients that were published between January 2012 and August 2016, the period following the publication of one of our studies on the same subject issued in 2012 in Supportive Care in Cancer, Volume 20, Issue 5 [1]. PubMed, Psychology and Behavioral Sciences Collection, and Scopus databases were chosen for this search as they contain research publications across a wide range of health professions in the field of oncology, and they are a leading source for journals related to clinical and psychosocial cancer care. The search strategy cross-references cancer (or oncology, or neoplasm) and body image and assessment (or questionnaire, or scale, or inventory, or measure, or psychometrics).
After identifying and classifying the relevant publications, a database search by tool name was performed. Finally, a manual search of the reference lists of the selected papers was performed to identify any further relevant publications.
Data extraction
The papers we identified were categorized according to which instrument they refer to. For each instrument, we identified the aspects of the considered body image, the number of items, the response format, the enrolled sample, the type of tested validity (construct, convergent, discriminant, criterion, cross-cultural, linguistic equivalence, feasibility, and positive response proportion), the type of verified reliability (internal consistency, temporal stability), and tool availability.
Assessing the construct validity consists of determining the underlying factorial structure of the tool. Convergent validity refers to the correspondence of the instrument to other already validated measures that assess the same construct. Discriminant validity consists of the tool’s ability to discriminate between different participants groups. Criterion validity refers to the tool’s ability to distinguish cases from non-cases. Cross-cultural validity evaluates the application of the tool to other cultural/linguistic contexts, whereas linguistic equivalence focuses on the equivalent value of the tool’s version in a language other than the original one. Feasibility considers the tool’s acceptance by compilers. Proportion of positive responses indicates the tool’s ability to capture the presence of all the aspects of the construct being considered. Internal consistency measures the items’ homogeneity, determining whether the scale consists of a single underlying construct. Temporal stability indicates whether the obtained scores remain constant over time.
For each tool, the available psychometric data were subsequently summarized by means of a code: “adequate”; “partial” (if available data were inconsistent and/or they were incomplete: for example, construct validity was tested only by exploratory factor analysis or was not tested for the original version but only for other linguistic versions, and internal consistency was provided for the total score only and not for each identified factor); or “non-available”. Cross-cultural validity was assessed as adequate when it was assessed for at least one additional cultural context other than the original one.
Results and discussion
The initial search (including manually retrieved articles) yielded 657 records. After excluding articles on the basis of the abovementioned criteria and due to duplication, we considered 23 articles [8–30] dealing with eight different tools: Appearance Schemas Inventory-Revised (ASI-R; [8]); Body Image after Breast Cancer Questionnaire (including its Chinese version; BIBCQ/BIBCQ-C; [9, 10]); Body Image and Relationship Scale ( including its Swedish version; BIRS/BIRS-S [11–13]); Body Image Scale (BIS; [14–21]); Body Image Screener for Cancer Reconstruction (BICR; [22]); Breast-Impact of Treatment Scale (including its Malay version; BITS/MBITS [23–25]); Measure of Body Apperception (MBA; [26–28]); and Sexual Adjustment and Body Image Scale (including its gynecologic version; SABIS/SABIS-g [28–30]).
Table 1 shows the characteristics and the tested psychometric properties for the identified body image tools as deducible by each selected paper. Table 2 summarizes the psychometric data available for each tool.
Of the eight identified instruments, only one (ASI-R) was borrowed from other populations (i.e. college students, [31, 32]); the remaining tools have been created specifically for oncology.
For all eight tools, validation studies reported involving breast cancer patients (at different stages of the experience of illness). A preliminary study of the BIS (see [14]) had involved a heterogeneous sample for the diagnosis of cancer patients, and in the literature, validation studies on ostomy patients [20] and patients undergoing surgery for colorectal cancer are available [21]. For the MBA, a study of the adaptation of the instrument to patients of both genders with head or neck cancer has been conducted [27]. Finally, for the SABIS, there exists an adaptation for gynecological oncological patients [30].
The body image aspects investigated by the eight tools are as follows: the investment in the body (two instruments: ASI-R, MBA); distress/stress/disturbance (three tools: BIS, BITS/MBITS, SABIS); and the concerns or issues (three instruments: BIBCQ/BIBCQ-C, BIRS/BIRS-S, BICR).
Half of the tools (BIS, BICR, MBA, SABIS) have a number of items not exceeding 10, and 2 instruments (ASI-R, BITS/MBITS) include a number of items between 11 and 20, whereas the remaining 2 tools (BIBCQ/BIBCQ-C, BIRS/BIRS-S) have more than 30 items.
The tools were made available in the selected publications. The only exceptions were the ASI-R, designed for other contexts and then validated for oncology, and SABIS that is available from the authors [28] (although the items and delivery of SABIS-G can be found in the relative publication [30]).
In none of the three articles relating to SABIS identified [28–30] were there indications about the scale of the response to the item.
Finally, the compilation time is only reported for the BIBCQ/BIBCQ-C.
Validity of the selected tools
Construct validity was assessed in seven of the eight instruments. In one case (ASI-R), authors recurred to a confirmatory factor analysis. In the remaining cases, the analysis was exploratory and sometimes (as in the case of BIRS, BIS, BITS/MBITS, SABIS) led to different results between studies. A confirmatory factor analysis was even conducted for BIBCQ-C, although in the original article on the BIBCQ [9] the instrument was described as multifactorial and this analysis was not reported.
Convergent validity was studied in six of the instruments (not shown for the ASI-R and BICR). In general, this was tested through correlations with similar psychological construct measures (including other measures of body image) and it documented its peculiarities.
For four instruments (BIBCQ/BIBCQ-C, BIS, BITS/MBITS, SABIS/SABIS-G), the discriminant validity was documented by verifying the ability to distinguish subsamples (usually stratified by type of treatment received, more or less invasive, and therefore impacting on the studied construct).
Only for the BICR are the data on the criterion validity available: in fact, for this tool a cutoff score was able to differentiate cases from non-cases.
The cross-cultural validity was verified for six of the instruments. Exceptions are the ASI-R (it must be remembered that this is the adaptation of a tool which is already in use for other populations) and the BICR. To date, the BIS is the tool about which we have the greatest number of cross-cultural studies available (it should be noted that 8 of the 23 selected studies address this instrument). In addition to data on cross-cultural validity, here the linguistic equivalence data were reported as verified for the BIRS/BIRS-S (English vs. Sweden), the BITS/MBITS (Malaysian vs. English), and the MBA (Spanish vs. English).
Information about the feasibility tool is available for BIBCQ/BIBCQ-C and the BIS, whereas the data for the positive response proportion are available only for the BIS.
Reliability of the selected tools
Internal consistency was verified in seven of the eight tools considered and it was satisfactory for all of them. An exception was BIRS/BIRS-S since in two of the three related papers internal consistency was verified for the entire questionnaire rather than for each subscale.
Temporal stability was adequate for BIBCQ/BIBCQ-C, BIS, BITS/MBITS, and SABIS/SABIS-G, but was tested only for the total score in BIRS/BIRS-S and was not tested in ASI-R and BICR.
Conclusions
In 2012, Supportive Care in Cancer published a review addressing relevance, application, and instruments of body image assessment for oncological settings. In that review, six different tools were presented, although a validation process (also if preliminary) had only been described for four of these. Today we have identified 23 papers related to the validation of eight different tools.
Although it cannot be argued that the validation process is exhaustive for any of the identified tools (future studies should be particularly directed to the definition of the criterion validity of these instruments), both the growing attention to the issue of the assessment of body image in oncology (which is very much deducible from the increased number of published instruments and the various works on cross-cultural validation) and the growing attention to the methodological aspects of evaluation (as seen in the different aspects of validity and reliability considered) must be acknowledged.
This review is intended to be of assistance as a rapid clinical method of assessing the instruments available in one’s own country. However, for researchers it shows the aspects that require further study, in particular (in addition to the already mentioned issue of criterion validity), the adaptation and use with other cancer populations as compared to breast cancer patients.
References
Annunziata MA, Giovannini L, Muzzatti B (2012) Assessing the body image: relevance, application and instruments for oncological settings. Support Care Cancer 20:901–907
Fingeret MC, Teo I, Epner DE (2014) Managing body image difficulties of adult cancer patients, lessons from available research. Cancer 120:633–641
Rhoten BA (2016) Body image disturbance in adults treated for cancer—a concept analysis. J Advance Nurs 72(5):1001–1011
Fingeret MC, Teo I, Goettsch K (2015) Body image: a critical psychosocial issue for patients with head and neck cancer. Curr Oncol Rep 17:422
Paterson CL, Lengacher CA, Donovan CA, Kip KA, Tofthagen CS (2016) Body image in younger breast cancer survivors, a systematic review. Cancer Nurs 39:e39–e58
Cash TF, Pruzinsky T (eds) (2002) Body images: a handbook of theory, research, and clinical practice. Guilford Press, New York
Cash TF, Smolak L (eds) (2011) Body image: a handbook of science, practice, and prevention. Guilford Press, New York
Chua AS, DeSantis SM, Teo I, Fingeret MC (2015) Body image investment in breast cancer patients undergoing reconstruction: taking a closer look at the Appearance Schemas Inventory-Revised. Body Image 13:33–37
Baxter NN, Goodwin PJ, Mcleod RS, Dion R, Devins G, Bombardier C (2006) Reliability and validity of the Body Image after Breast Cancer Questionnaire. Breast J 12:221–232
Zhang J, Zhu X, Tang L et al (2014) Psychometric features of the body image after breast cancer questionnaire-Chinese version in women with breast cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 39:73–77
Hormes JM, Lytle LA, Gross CR, Ahmed RL, Troxel AB, Schmitz KH (2008) The Body Image and Relationships Scale: development and validation of a measure of body image in female breast cancer survivors. J Clin Oncol 26:1269–1274
Larsson YH, Speck R, Schmitz KH, Johansson K, Gyllenstend AL (2014) The Body Image and Relationship Scale: a Swedish translation, cultural adaptation, and reliability and validity testing. Eu J Physiother 14:67–75
Vieira EM, dos Santos MA, Santos DB et al (2015) Validation of Body Image Relationship Scale for women with breast cancer. Rev Bras Ginecol Obstet 37:473–479. doi:10.1590/SO100-720320150005354
Hopwood P, Fletcher I, Lee A, Al Ghazal S (2001) A body image scale for use with cancer patients. Eur J Cancer 37:189–197
Moreira H, Silva S, Marques A, Canavarro MC (2010) The Portuguese version of the Body Image Scale (BIS)—psychometric properties in a sample of breast cancer patients. Eur J Oncol Nurs 14:111–118
Khang D, Rim HD, Woo J (2013) The Korean version of the body image scale-reliability and validity in a sample of breast cancer patients. Psychiatry Investig 10:26–33
Songtish D, Hirunwiwatkul P (2013) Development and validation of the body image scale among Thai breast cancer patients. J Med Assoc Thail 96(Suppl 1):S30–S39
van Verschuer VM, Vrijland WW, Klem TM (2015) Reliability and validity of the Dutch-translated Body Image Scale. Qual Life Res 24:1629–1633
Gómez-Campelo P, Bragado-Álvarez C, Hernández-Lloreda MJ, Sánchez-Bernardos ML (2015) The Spanish version of the Body Image Scale (S-BIS): psychometric properties in a sample of breast and gynaecological cancer patients. Support Care Cancer 23:473–481
Karayurt Ö, Edeer AD, Süler G et al (2015) Psychometric properties of the Body Image Scale in Turkish ostomy patients. Int J Nurs Knowl 26:127–134
Whistance RN, Gilbert R, Fayers P et al (2010) Assessment of body image in patients undergoing surgery for colorectal cancer. Int J Color Dis 25:369–374
Fingeret MC, Nipomnick S, Guindani M, Baumann D, Hanasono M, Crosby M (2014) Body image screening for cancer patients undergoing reconstructive surgery. Psycho-Oncol 23:898–905
Frierson GM, Thiel DL, Andersen BL (2006) Body change stress for women with breast cancer: the Breast-Impact of Treatment Scale. Ann Behav Med 32:77–81
Zainal NZ, Shuib N, Bustam AZ, Sabki ZA, Guan NC (2013) Reliability and validity of the Malay Version of the Breast-Impact of Treatment Scale (MVBITS) in breast cancer women undergoing chemotherapy. Asian Pac J Cancer Prev 14:463–468
Carver CS, Pozo-Kaderman C, Price AA et al (1998) Concern about aspects of body image and adjustment to early stage breast cancer. Psychosom Med 60:168–174
Perczek R, Carver CS, Price AA, Pozo-Kaderman C (2000) Coping, mood, and aspects of personality in Spanish translation and evidence of convergence with English versions. J Pers Assess 74:63–87
Jean-Pierre P, Fundakowski C, Perez E et al (2013) Latent structure and reliability analysis of the measure of body apperception: cross-validation for head and neck cancer patients. Support Care Cancer 21:591–598
Dalton EJ, Rasmussen VN, Classen CC et al (2009) Sexual Adjustment and Body Image Scale (SABIS): a new measure for breast cancer patients. Breast J 15:287–290
Özalp E, Karslıoğlu EH, Aydemir Ö et al (2015) Validating the Sexual Adjustment and Body Image Scale (Sabis) with breast cancer patients. Sex Disabil 33:253–267
Ferguson SE, Urowitz S, Massey C et al (2012) Confirmatory factor analysis of the Sexual Adjustment and Body Image Scale in women with gynecologic cancer. Cancer 118:3095–3104
Cash TF (2003) Brief manual for the Appearance Schemas Inventory-Revised. http://www.body-images.com
Cash TF, Melnyk SE, Hrabosky JI (2004) The assessment of body image investment: an extensive revision of the Appearance Schemas Inventory. Int J Eat Disord 35:305–316
Acknowledgements
The Authors wish to thank Ms. Anna Vallerugo, MA, for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Muzzatti, B., Annunziata, M.A. Body image assessment in oncology: an update review. Support Care Cancer 25, 1019–1029 (2017). https://doi.org/10.1007/s00520-016-3538-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3538-y