Abstract
Background
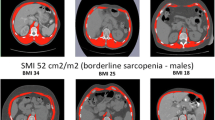
Single cross-sectional area of muscle at the third lumbar vertebra (L3MA) is gold standard to estimate skeletal muscle mass (SMM), and L3 muscle index (L3MI, L3MA/height2) is used to determine sarcopenia. The purposes of this study were to evaluate the relationship between SMM indices determined by routine chest CT and L3MI in patients with small-cell lung cancer (SCLC) and to suggest chest CT-derived diagnostic criteria for sarcopenia.
Methods
Area of pectoralis muscles at the aortic arch (PMA) and at L1 (L1MA) was retrospectively measured on chest CT images of 90 consecutive SCLC patients. Pearson’s correlation and multiple linear regression analysis were used to assess relationships between L3MI determined by PET/CT and pectoralis muscle index (PMI) and L1 muscle index (L1MI) determined by chest CT.
Results
The correlation between L1MI and L3MI was stronger than that between PMI and L3MI (r = 0.851 vs. r = 0.447, p < 0.001). Multivariable regression analysis showed that L1MI was the only significant predictor of L3MI; L3MI = 0.963 × L1MI + 10.336 (R 2 = 0.689, p < 0.001) for male and L3MI = 0.772 × L1MI + 16.518 (R 2 = 0.777, p < 0.001) for female. Using this relationship, estimated cutoffs of L1MI for sarcopenia were 46 cm2/m2 for male and 29 cm2/m2 for female (L3MI cutoffs for sarcopenia are 55 cm2/m2 for male and 39 cm2/m2 for female). The sensitivity and specificity of L1MI cutoffs to determine sarcopenia were 98.2 and 100 %, respectively.
Conclusions
Chest CT-determined L1MI is highly correlated with L3MI in SCLC patients. L1MI, as determined by chest CT, could be used to determine the presence of sarcopenia with suggested cutoffs of 46 cm2/m2 for men and 29 cm2/m2 for women.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcopenia, the loss of skeletal muscle mass (SMM), is an important age-related health problem in the elderly, because it contributes to functional decline, disability, injury, and mortality. The clinical importance of sarcopenia is also being increasingly recognized in oncologic patients as a component of cancer cachexia syndrome [1, 2]. Sarcopenia is a prevalent condition in cancer patients and is associated with functional impairment, increased risk of chemotherapy-related toxicities, and reduced survival [3–6]. The prognostic significance of sarcopenia in the oncology setting emphasizes the need for identifying its presence and for effective therapeutic interventions to increase SMM.
The quantification of SMM is important in this context. Regional analyses of body compositions at specific abdominal landmarks strongly predict whole-body fat mass (r = 0.842–0.963, p < 0.001) and SMM (r = 0.712–0.924, p < 0.001), and single cross-sectional area of SMM at the third lumbar vertebra (L3) was found to be most correlated with total body SMM (r = 0.924, p < 0.001). Therefore, L3 muscle area (L3MA) as determined by computed tomography (CT) or by magnetic resonance imaging (MRI) is considered reference standards for estimating total body SMM [7–10], and gender-specific cutoffs of height-adjusted L3MA, L3 muscle index (L3MI, L3MA/height2), are currently used to define sarcopenia in cancer cachexia trials [1]. Although chest CT is the standard imaging modality used to diagnose and follow up lung cancer, it rarely extends to the L3 level, which precludes its use for assessing the status of sarcopenia in lung cancer patients. For this reason, one study evaluated pectoralis muscle area (PMA) to define sarcopenia in patients with small-cell lung cancer (SCLC) [11]. Although L1 is covered during chest CT examinations and muscle area at the first lumbar vertebra level (L1MA) was found to show a high correlation (r = 0.903, p < 0.001) with total body SMM in healthy subjects [7], the predictive values of chest CT-determined SMM indices for determining sarcopenia have not been assessed. Accordingly, the primary objectives of the present study were to evaluate relationships between specific chest CT-determined SMM indices and L3MI in SCLC patients and to suggest diagnostic chest CT criteria for sarcopenia.
Patients and methods
Patients
The radiologic database and medical record system at Gachon University Gil Medical Center (Incheon, Korea) were searched for patients with newly diagnosed, pathologically proven SCLC that underwent a baseline chest CT scan and a PET/CT scan on the same week from January 2010 to November of 2014. Heights and weights were measured, and functional statuses were recorded at first visit to our oncology department. Body mass index (BMI) was defined as weight divided by height squared (kg/m2), and BMI values were categorized as underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), or obese (≥25 kg/m2) [12].
SCLC was classified as limited or extensive. Limited stage was defined as American Joint Committee on Cancer (AJCC) stages I to III, which can be safely treated by definitive radiation therapy [13]. The institutional review board of our hospital approved this retrospective study and waived the requirement for informed patient consent.
Image analysis
Routine chest CT was performed using one of the three helical scanners, that is, a 64-slice, a dual-source 64-slice CT scanner, or a second-generation dual-source MDCT scanner (SOMATOM Sensation 64, SOMATOM Definition, Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany). All patients fasted for at least 6 h beforehand, and post-contrast images were obtained within 30–40 s of commencing an injection of 120 ml of nonionic iodinated contrast material (Ultravist 300 mg/ml, Schering AG, Berlin, Germany) administered through an antecubital vein at 3–4 ml/s. Scanning was performed from the thoracic inlet to the middle portion of the kidneys using the following scanning parameters: 170 mAs, 120 kVp, collimation of 64 × 0.6 mm, scanning range 35 cm, pitch 0.75, and gantry rotation time 330 ms.
Quantitative assessments of muscle areas were performed using commercially available software (Terarecon 3.4.2.11, San Mateo, CA, USA) by a subspecialty-trained chest radiologist. Single cross-sectional PMAs just above the aortic arch and L1MA were quantitatively assessed. To determine PMAs, the observer visually identified the aortic arch and then selected the first axial image just superior to the arch, and the area of bilateral pectoralis major and minor muscles on the anterior chest wall was measured. Tissue cross-sectional areas (cm2) in slices were computed automatically by summing appropriate pixels using the CT Hounsfield unit (HU) range −29 HU to 150 HU for skeletal muscle. After applying threshold methods using predefined HU thresholds to slices, boundaries between different tissues were corrected manually when necessary. SMM index was defined as the CT-determined cross-sectional area of muscle divided by height squared (cm2/m2) (e.g., L1 muscle index (L1MI); L1MA/m2).
The L3MA measured on PET/CT images at the time of SCLC diagnosis served as reference standards. The integrated CT (Siemens Medical Systems, Erlangen, Germany) used was equipped with lutetium oxyorthosilicate crystal PET detectors and six slice detectors. Imaging parameters were as follows: 130 kVp, 110 mAs, 2-mm pitch, 1-s tube rotation, and a slice thickness of 5 mm, which matched the section thickness of PET images.
Statistical analysis
Descriptive statistics are reported as proportions or means with standard deviations (SDs). Pearson’s chi-squared test or Fisher’s exact test was used to compare categorical variables between men and women, whereas continuous variables were compared using the Student’s t test or the Mann-Whitney U test. Pearson’s correlation analysis was used to assess relationships between L3MI as determined by PET/CT and continuous variables, such as L1MI, PMI, age, height, and BMI. To determine differences between correlation coefficients, correlations were transformed using Fisher’s z transformation followed by the t test. In order to find the important predictors for multivariable model, stepwise method with F statistic was applied where candidate variables with a p value <0.05 were entered and those with a p value >0.10 were removed from the model. Bland-Altman plots were used to assess agreements between two measurements. Statistical significance was calculated at the 95 % confidence interval (p < 0.05), and the analysis was performed using SPSS for Windows ver. 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the study population
Ninety consecutive patients were included in the present study, and their characteristics are summarized in Table 1. Mean patient age was 69.1 ± 9.4 years, and most (n = 75, 83.3 %) were male. Of the 90 patients, 62 (68.9 %) had extensive disease at first presentation. Average BMI was 22.4 ± 3.6 kg/m2, and 16.7 % of the 90 study subjects were underweight (n = 15). Mean value ± SD of L3MI, L1MI, and PMI were 49.4 ± 9.2, 40.6 ± 8.3, and 15.6 ± 4.0 cm2/m2, respectively. SMM indices, height, and weight were significantly greater for men.
Relationship between L3MI and subject characteristics and SMM indices
Correlations between L3MI and variables were summarized in Table 2. In the 90 study subjects, weight, BMI, PMI, and L1MI were positively correlated with L3MI (r = 0.447–0.851, p values <0.001), but age was negatively associated with L3MI (r = −0.366, p < 0.001). The strongest correlation was observed between L1MI and L3MI (r = 0.851). Furthermore, L1MI was better correlated with L3MI than PMI (r = 0.447, p < 0.001) in the study subjects.
Estimated L1MI cutoffs to determine sarcopenia
According to multivariable regression analysis using age, weight, and BMI as co-variables, L1MI was the only significant predictor of L3MI; L3MI = 0.963 × L1MI + 10.336 (R 2 = 0.689, p < 0.001) for male and L3MI = 0.772 × L1MI + 16.518 (R 2 = 0.777, p < 0.001) for female (Fig. 1). Bland-Altman analysis did not disclose any significant bias between predicted and measured L3MI (Fig. 2).
L3MI cutoffs for sarcopenia are 55 cm2/m2 for men and 39 cm2/m2 for women according to a recently published consensus definition of cancer cachexia [1]. Using the abovementioned regression model, the L1MI cutoffs for sarcopenia were 46 cm2/m2 for men and 29 cm2/m2 for women. Using these cutoffs, the sensitivity, specificity, and accuracy of L1MI to determine sarcopenia were 98.2, 100, and 98.9 %.
Discussion
Cachexia has been defined as a complex metabolic syndrome associated with underlying illness and characterized by muscle depletion with or without fat tissue loss [1]. The classical clinical feature of cachexia is weight loss, but body weight changes do not precisely reflect body composition change, because muscle wasting is obscured by changes in body fat levels and because weight loss is uncertain in patients with a large tumor mass or exhibiting fluid collection, such as pleural effusion, ascites, or body edema.
A wide range of techniques, including MRI, CT, dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA) can be used to assess SMM [14], and considerations, such as cost, availability, accuracy, and ease of use, largely determine whether techniques are better suited to clinical practice or research. CT and MRI are considered precise imaging modalities that can separate fat and muscle from other soft tissues, which is the reason why these methods are considered gold standards for estimating body compositions in research. Many investigators have focused on specific landmarks in the abdominal region in CT and MRI studies, and single cross-sectional areas are known to be well correlated with whole-body SMM in healthy individuals [7–10]. Although L3 is considered as a standard skeletal landmark for the quantification of whole-body SMM since it has been shown to exhibit the strongest correlation, cross-sectional area at L1, like CT images at the L3 level, also contains visceral, subcutaneous, and inter-muscular adipose tissue, psoas, and paraspinal muscles, and it has been shown that a strong correlation exists between L1MA and whole-body SMM [7].
Although CT is viewed as a gold standard method for assessing body composition, its use in noncancer populations is limited by high levels of radiation exposure, cost, and availability and accessibility shortcomings. However, these drawbacks do not apply in oncology settings as patients undergo routine CT examinations for diagnosis and to monitor disease progression. A recently published consensus definition of cancer cachexia established sarcopenia as a key diagnostic criterion and suggested L3MI < 55 cm2/m2 and <39 cm2/m2 as a CT diagnostic criteria for sarcopenia in men and women, respectively [1]. However, lung cancer patients usually undergo chest CT, which does not extend to L3, the standard skeletal landmark used to determine the presence of sarcopenia.
The present study is the first to compare SMM indices of specific regions by chest CT using L3MI (the standard currently used to evaluate sarcopenia) as the reference standard. The study revealed a linear relationship between routine chest CT-determined PMI and L1MI and PET/CT-determined L3MI and a better correlation between L1MI and L3MI than between PMI and L3MI. Furthermore, multivariable regression analysis showed that L1MI was the only independent predictor of L3MI, and using the derived regression equation between L1MI and L3MI, we were able to suggest L1MI cutoffs for sarcopenia.
Pectoralis muscle has the merit of being easily demarcated and quantified due to its anatomical features on chest CT images, and furthermore, prognosis was poor in male SCLC patients with a low PMA and an elevated neutrophil-to-lymphocyte ratio [11]. In a recent study [15], a moderate correlation (r = 0.665, p < 0.001) was observed between CT-determined PMA and BIA-determined total body SMM in healthy subjects, and this association remained significant after adjusting for sex, weight, height, and age. Further large-scale study is warranted to evaluate the usefulness of PMA for quantifying SMM and for predicting prognosis in oncologic patients.
In conclusion, the present study shows that chest CT-determined L1MI is highly correlated with L3MI in patients with SCLC. Using the derived regression equation, we suggest L1MI cutoffs of 46 cm2/m2 for men and 29 cm2/m2 for women to determine sarcopenia. In our opinion, chest CT-determined L1MA could serve as a means of determining the presence of sarcopenia for patients with thoracic malignancy in future studies.
Author contributions
All authors participated in the concept/design, data interpretation, drafting, and critical revision of this article. The statistical analysis was conducted by Y. S. Kim.
References
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB (2013) Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 47:861–870
Fukushima H, Yokoyama M, Nakanishi Y, Tobisu K, Koga F (2015) Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 10:e0115895
Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM (2015) Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol 10:1795–1799
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB (1985) Heshka S (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 85:115–122
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Go SI, Park MJ, Song HN, Kang MH, Park HJ, Jeon KN, Kim SH, Kim MJ, Kang JH, Lee GW (2016) Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 24(5):2075–2084
Consultation WHOE (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Kalemkerian GP, Gadgeel SM (2013) Modern staging of small cell lung cancer. J Natl Compr Cancer Netw 11:99–104
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Kim YS, Kim EY, Kang SM, Ahn HK, Kim HS (2015) Single cross-sectional area of pectoralis muscle by computed tomography—correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin Physiol Funct Imaging. doi:10.1111/cpf.12333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no proprietary, commercial, or financial interests that could be construed to have inappropriately influenced this study.
Rights and permissions
About this article
Cite this article
Kim, E.Y., Kim, Y.S., Park, I. et al. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer 24, 4721–4726 (2016). https://doi.org/10.1007/s00520-016-3321-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3321-0