Abstract
Purpose
Glioblastoma (GBM) patients have many palliative care (PC) issues. To date, there are no studies examining the prospective usage of validated PC assessment tools as patient reported outcome measures for GBM patients.
Methods
GBM patients’ PC issues were assessed from diagnosis to death or for at least 12 months every 7 weeks (±8 days) using semi-structured interviews and the Hospice and Palliative Care Evaluation (HOPE, including Eastern Cooperative Oncology Group (ECOG) performance status, 17 items) and the Palliative Outcome Scale (POS, 11 items). Data from patients who died within 12 months of the last patient’s enrollment were evaluated using summarizing content analysis, visual graphical analysis (VGA), and linear mixed models for repeated measures.
Results
Nineteen of 33 patients screened were enrolled; two dropped out and four were still alive at the end of the study. The remaining 13 were assessed at 59 points until death (time range 4–68 weeks; 1–10 contacts per patient; assessment: self, 33; joint, 8; external, 18). VGA of the HOPE and POS data, including all 1,652 assessed item data, showed consistent trajectory profiles for 14 of 28 items: 10 were increasing (meaning symptom worsening) and comprised predominantly psychosocial issues and care dependency. Type of assessment partly interacted with time, however, not qualitatively so. Analysis of semi-structured interviews revealed delayed interactions with PC/hospice services and numerous neuropsychiatric problems not detected by HOPE and POS.
Conclusions
Prospective self-assessment of GBM patients’ PC issues is feasible. However, disease progression may necessitate further, external assessment. Modification of existing PC assessment tools is needed to detect GBM-specific issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common malignant brain tumor in adults. With a median survival of about 14 months, GBM patients have the poorest prognosis of all malignant brain tumor patients [1]. This rather short disease course means that serious, life-changing symptoms (neurological, cognitive/psychological, nutritional, respiratory, and urinary) [2] and needs (medical, nursing, caregiver, emotional, and financial) [3, 4] evolve rapidly. Various aspects of quality of life including emotional, physical, cognitive and social functioning, and spiritual well-being are often substantially affected already at diagnosis [5]. Previous retrospective studies investigating issues faced by end-of-life glioma patients have found that their symptom burden is high, including neuropsychiatric symptoms and psychosocial and logistical problems [3, 4, 6–9]. However, GBM patients are significantly less likely to receive palliative care than other cancer patients: Only 2–3 % of admissions to specialized palliative care units [3, 10] or to palliative care services [11] suffer from a primary brain tumor.
The rapidly evolving symptoms and needs in GBM lead to the hypothesis that regular, validated palliative care (PC) assessments as patient-reported outcome measures (PROMs) from diagnosis to death could help tailor palliative care to GBM patients during disease progression.
The aim of this pilot feasibility study was to test whether (a) GBM patients are capable of regular self-assessment of their symptoms and needs during disease progression, (b) classical, validated PC assessment tools cover all PC issues relevant to GBM patients, and (c) by using our assessment method of validated PC assessment tools combined with a semi-structured interview, more insight into symptoms and needs throughout GBM progression could be gained.
Methods
A prospective, hospital-based, cohort, pilot feasibility study was conducted with GBM patients. The recruiting period was 11 months. The observation period spanned 12 months after the last patient was included.
Patients
Patients from three departments (Center of Neurosurgery and Department of Neurology, University Hospital of Cologne, and the Department of Neurology, University Hospital Bonn) were screened for inclusion directly after initial or suspected initial diagnosis of GBM. Due to low recruitment rates, the screening criteria were amended 8 months into the recruitment phase to allow screening of GBM patients not only directly after their initial diagnosis. Patients were not included if final histology revealed an etiology other than GBM, they were unable to give informed consent, or they declined to participate. All participants gave written consent. The Ethics Committee of the University Hospitals of Cologne (ethic approval: No. 06-243) and Bonn (ethic approval: No. 081/07) approved the study.
Data collection
Assessments were done by the PC interviewer (MA, physician working as an oncology resident while a doctoral student in palliative care), initially, in-person, with follow-up assessments done via telephone. An assessment interval of every 6 weeks until death, dropout, or end of observation period was tested for feasibility. If self-assessment was not possible any longer, external assessment by caregivers was allowed.Footnote 1
Each assessment consisted of two parts: In order to elicit additional issues not covered by standardized assessment tools, the interviewer first conducted a semi-structured interview asking open questions on current disease status, treatment, problems, burdensome symptoms, and wishes for a better care (this could be additional therapies, home care, palliative/hospice care, and end-of-life care). In the final phone interview, caregivers were asked in a semi-structured interview to describe the death of the patient (setting, circumstances, etc. at the time shortly before the patient’s death) and to summarize successful and unsuccessful aspects of the patient’s individual care over the course of their illness until their death. The second part consisted of two structured PC assessments: the core documentation of the German Hospice and Palliative Care Evaluation initiative (HOPE = Hospiz- und Palliativerhebung) [12], the HOPE symptom and problem checklist (HOPE-SP-CL) including the Eastern Cooperative Oncology Group (ECOG) performance status scale [13, 14], and the validated German version of the Palliative Outcome Scale (POS) [15, 16]. This study focused on the assessment of PC issues and whether validated PC assessment tools cover all PC issues relevant to GBM patients. Therefore, in this study, we utilized HOPE and POS combined with semi-structured interviews precluding further validated quality of life assessment tools. The utilized HOPE questionnaires collect personal data including social situation, stage of disease, symptom burden, current medication, measures and activities carried out to support the patient, and his/her satisfaction with end-of-life care [13, 14]. In the HOPE-SP-CL, symptoms and problems are assessed using a four-point grading scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe). The ECOG performance status is a five-point grading scale ranging from 0 (normal activity) to 4 (care dependent, totally confined to bed).
The POS consists of 10 items, one open question and the sum score which assess physical symptoms and emotional, psychological, and spiritual needs experienced over the previous 3 days. Each of the first 10 POS items consists of a question and five possible answers; for each item, answers are presented in both numeric and narrative forms; a Likert scale is used to score these answers ranging from zero (best) to four (worst). The numeric ratings yield both individual item scores and overall profile scores (questions number 1–10; ranging from 0 to 40). The 11th question provides patients the opportunity to list, in their own words, the main problem(s) experienced over the previous 3 days [15, 16].
The interviewer noted the assessment type (self-, joint, or external assessment). Field notes were made; interviews were not recorded.
Analysis
Only the data of those patients who were studied until death within the observation period were analyzed. Longitudinal data were analyzed beginning from death and working “backward”. Patients’ symptom/problem burden as recorded by HOPE and POS was described by summary statistics (mean and standard deviation) for each investigation point. Trajectories for single items (HOPE 17 items including ECOG, POS 11 items including sum score) were plotted for each patient (y-axis: symptom/problem scale; x-axis: number of contacts). Each trajectory represents the development of one item for a single patient during disease progression. Individual trajectories were analyzed using visual graphical analysis (VGA), which facilitates the identification of common patterns for single items and thus the development of categories of trajectories [17]. This method helped to describe whether or not specific symptoms/needs developed consistently during disease progression (defined as >50 %, i.e., a simple majority, of assessment curves showing the same trajectory profile for a specific symptom/need). Linear mixed models for repeated measures (MMRM with effects time [AR1 covariance structure], type of assessment, and their interaction) were fitted to evaluate time trends (i.e., using linear contrasts), adjusted for change in type of assessment. Statistical analyses were performed using Excel (Microsoft Corp., Redmond, WA, USA) and SPSS (IBM Corp., Armonk, NY, USA).
The field notes of the semi-structured interviews were analyzed via summarizing content analysis [18]. Interviews were analyzed for evolving categories of neuropsychiatric symptoms, additional therapies, and circumstances of death. Thematic units concerning the three topics were analyzed and categorized using the constant comparative method (HG, MA, and MG) [19] and were multi-professionally discussed among authors (HG, MA, MG, MH, and RV) [20]. This method resulted in gradual refinement from the first codes to preliminary categories and then to categories on a higher abstraction level that could be applied to all data.
Results
Sample
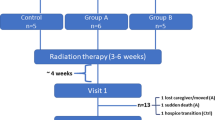
Thirty-three patients were screened for the study. Of these, 14 were excluded due to a final histology indicating etiology other than GBM (four), inability to give consent (five), or declining to participate (five). Of the 19 enrolled patients, two dropped out as they and/or their caregivers found it too difficult to speak about the illness and associated symptoms. The remaining 17 patients (or their caregiver instead of them, if necessary) were assessed every 7 weeks (±8 days). The planned assessment interval of 6 weeks was not achieved due to scheduling conflicts (other appointments or holidays) or requests to reschedule (due to discomfort or tiredness). The regular assessment started early following diagnosis (1.5–5 weeks), except for two patients whose diagnoses dated back 12 weeks and 15 months (per study amendment), respectively. Of the 17 enrolled patients, four were still alive at the end of the observation period; their data were cut from the final analysis (Fig. 1). At diagnosis, two patients had advance directives; one of these also had a health care proxy. During the course of the study, seven additional patients obtained health care proxies, and two additional patients obtained advance directives. For characteristics of the 13 patients, see Table 1.
Process of assessment
Mean assessment duration was 27 min (range 12 to 80 min). The 13 patients were assessed at 59 points before death. Time range of observation was 4–68 weeks with the number of contacts as follows (number of patients in brackets): 1 contact (two), 2 contacts (one), 3 contacts (two), 4 contacts (two), 5 contacts (one), 6 contacts (two), 7 contacts (two), and 10 contacts (one). Self-assessment was possible in 33 of 59 instances (56 %); 8 (13.5 %) and 18 (30.5 %) assessments were done jointly or by caregiver(s), respectively (Fig. 2). Patients were deemed “unable” to take part in an assessment if this was stated by patients themselves or by their caregivers; no objective criteria were utilized. Reasons emerging for joint or external assessment were increasing physical, psychiatric, or neuropsychological impairment. External assessment predominated self-assessment only at the last assessment before death. The mean duration of the final assessment with caregiver(s) after patients’ deaths was 11 min (range 3 to 54 minutes).
Superimposed trajectories of selected items for HOPE and POS for the 13 patients analyzed. Longitudinal data were analyzed beginning with death (i.e., in reverse). Individual trajectories were slightly jittered (±0.2 at maximum) to improve presentation, and estimated marginal means (dark grey) with corresponding 95 % confidence regions (light grey) were calculated from MMRM analysis to evaluate trends (linear contrasts). The type of assessment is visualized by different shapes, i.e., circle = self-assessment, square = joint assessment, and cross = external assessment. If type of assessment interacted with time, estimated marginal means were plotted by type, i.e., red = self-assessment, green = joint assessment, and blue = external assessment
Symptom/Problem burden according to HOPE and POS
Tables 2 and 3 present the average symptom/problem burden assessed by HOPE and POS, respectively, during disease progression (total 28 items, 1,652 data points). Contacts -10 to -7 are not shown in either table as at contacts -10 to -8 only one patient and at contact -7 only three patients were still alive. Four symptom/problem development trajectory profiles (increasing, fluctuating, steady, and decreasing) were identified via VGA.
With respect to HOPE (Table 2), consistent trajectory profiles for at least seven of the 13 patients (>50 %) were found for the following items: ECOG, constipation, tiredness, assistance with activities of daily living (ADLs), overburdening of family burden (increasing trajectory profile, i.e., deterioration), and wound care and vomiting (steady trajectory profile at zero).
With respect to POS (Table 3), consistent trajectory profiles for at least seven of the 13 patients (>50 %) were found for other symptoms (than pain, e.g., nausea, dyspnea, cough, and constipation), family anxiety, support (i.e., inability to share feelings with friends/relatives), life worthwhile, and POS total score (increasing trajectory profile) and anxiety (fluctuating trajectory profile on a level above zero) and wasted time (for treatment-related appointments) (steady trajectory profile at zero). VGA revealed inconsistent trajectory profiles for all other symptoms/problems.
In summary, the main palliative care issues during GBM disease progression as revealed by HOPE and POS were decreasing levels of emotional support, decreasing feeling that life was worthwhile, alongside increasing physical impairment, care dependency burden, family burden, and patient/family anxiety.
Statistically significant differences between self-, joint, and external assessment were found for the following items (see footnote in Tables 2 and 3)—HOPE: ECOG, wound care, overburdening of family, and assistance with ADLs; POS: pain and wasted time, with joint/external assessments scoring higher (worse) than self-assessments. Regardless of the type of assessment, trends essentially remained consistent, i.e., showing similar behavior in the proximity of death. Figure 2 shows how important items developed over time.
Summarizing content analysis of semi-structured interviews
Neuropsychiatric symptoms
Patients experienced numerous neuropsychiatric symptoms (Table 4). All patients developed at least one neuropsychological symptom during disease progression. Other neuropsychiatric symptoms occurring in more than 50 % of patients included the effects of intracranial pressure, epileptic seizures, paresis, motor coordination disorder, quantitative disturbance of consciousness, apathy, symptoms of delirium, symptoms of depression, and anxiety and experienced loss of autonomy.
Additional therapies
Additional therapies were rarely engaged. Three patients received speech therapy; two received physiotherapy; none received occupational therapy; one received support from a psychooncologist.
Circumstances of death
Acute care hospital
Despite having highly engaged caregivers, recurrent crises during disease progression and especially rapid deterioration at the end of life led to repeated hospitalizations, and ultimately, five patients who had wished to die at home or in a hospice died in acute care hospitals.
Hospice and nursing home
Five patients, two of whom wished to die at home, died in a hospice (four) or a nursing home (one). All five were able to stay at home as long as possible thanks to their caregivers, even when diverse crises, mostly due to severe neuropsychiatric symptoms, and/or care dependency, led to temporary stays in a hospital. As the disease progressed, caregivers felt exhausted and experienced relief once patients were offered a place in a hospice/nursing home. Only patients with advanced-stage disease were offered a place in a hospice. The patient who died in a nursing home would have gone to a hospice; however, it was too far away from home.
Home
Dedicated caregivers, thorough advance care planning, and crisis management using short hospital stays (as sufficient outpatient care was not available in these crises) enabled three patients who truly wished to die at home to do so.
Discussion
This hospital-based, cohort, pilot feasibility study investigated for the first time the feasibility of the prospective usage of the validated PC assessment tools as PROMS for GBM patients from diagnosis until death.
Feasibility of PROMS
Self-assessment was achieved in 56 % of assessments. Increasing impairment due mostly to neuropsychiatric symptoms hindered self-assessment in the remainder of the assessments. Missing data were circumvented by allowing joint or external assessment by caregivers. The need for and frequency of joint- or external assessment increased as the disease progressed, which partly influenced measured outcomes (six of 28 items) in a statistically significant manner. However, given that trends essentially remained consistent, we assume that our interpretation of the outcome data of this study is not seriously biased. Though external assessments have been found to match self-assessments in several aspects of patients’ health-related quality of life [21], there is some evidence suggesting that ratings diverge when patients are cognitively impaired [22]. There is also evidence that caregivers might report more problems than patients do regarding personal and psychological items like “is life worthwhile” [23]. In future studies, an increasing need for external assessment during GBM progression is to be anticipated.
Dropout and feasibility of assessment
The dropout rate of 10.5 % (2/19) was low [24, 25] and indicates that prospective PC assessment was well accepted overall. The targeted assessment interval of 6 weeks was not reached completely due to patient-related reasons, but an interval of 7 weeks was feasible, if done by telephone. Requiring in-person follow-up assessments could result in a higher dropout rate, as increasing impairment could impact patients’ ability to travel. Alternatively, the assessment could be conducted in the patient’s home. While this would be more resource intensive, it would also allow objective medical assessment of the patient’s ability to participate. Allowing joint or external assessment in lieu of self-assessment, when necessary, ensured that at least proxy data were collected.
Our aim to study GBM patients’ palliative care issues until death was possible in 13 of 17 patients, as four patients were still alive at the end of the observation period. Thus, future studies should utilize a longer observation period. In two of the 13 analyzed patients, disease progression was so rapid that only one assessment was possible.
Recruitment
Seventeen percent (5/29) of those with a final diagnosis of GBM declined to participate; this is comparable to other PC studies frequently experiencing recruiting difficulties and high dropout rates [24, 25]. Another 17 % (5/29) could not consent due to cognitive and/or physical impairment already at diagnosis. Such patients are particularly challenging for all providers and are therefore of special interest, and future studies should consider ways to include them. Despite the study amendment, the sample size did not increase. One reason for this may be that the current study was not run by an official study office coordinating recruitment.
Palliative care assessment tools and insight into palliative care issues of GBM patients
The standard PC assessment tools, HOPE and POS, were developed based on PC patients suffering from cancer diagnoses in general, not specifically GBM [13–15]. Consistent with previous findings [3], in the current study, those symptoms assessed by HOPE in particular played a prominent role and mostly worsened with disease progression, leading to increased care dependency and consequently to a greater burden for caregivers. The results of POS in the current study reflect an overall accumulation of impairments leading to questioning whether life was worthwhile as well as to increasing family anxiety. On these points, standard PC assessment tools seem to be appropriate for GBM patients. However, other symptoms assessed by HOPE and POS [13–16], like dyspnea, pain, nausea, vomiting, and loss of appetite, which are common in other types of cancer, were of minor importance in the current study. Rather, data gathered from the semi-structured interviews in the current study found that the GBM patients studied developed a variety of neuropsychiatric symptoms, which, in part, even had a higher incidence than that presented elsewhere [3, 6, 8, 9]. This might be attributed to the fact that our study participants all suffered from the highly malignant brain tumor GBM and most were repeatedly interviewed. Thus, the standard PC assessment tools HOPE and POS fail to capture certain aspects of the GBM experience. The neuropsychiatric symptom burden is not reflected at all (e.g., motor or sensory disturbances, epileptic seizures, and neuropsychological symptoms) or is not adequately reflected (e.g., delirium). General items like “disorientation/confusion,’ “tension,” or “feeling depressed” did not show as convincing results as that in the semi-structured interview, which offered patients and/or caring relatives the chance to describe problems in their own words instead of being restricted to fixed terms that could be misunderstood [26]. Hence, future studies should also check for neuropsychiatric symptoms in GBM patients possibly with the use of the European Organization For Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire, Brain Module (QLQ-BN 20) [27], a validated tool for assessing neurological symptoms in patients with brain tumors, and the Neuropsychiatric Inventory (NPI) Worksheet, which was originally developed to assess neuropsychiatric symptoms in dementia patients [28].
Advance care planning
Thirty-one percent of patients in the current study obtained advance directives during the course of GBM disease, and 62 % obtained health care proxies. While this number can clearly be improved, as worsening of physical and cognitive performance status of GBM patients requires a thorough advance care planning, it is higher than previously reported [8]. It is possible that repeated interviews in the current study positively influenced GBM patients’ advance care planning.
Family caregivers
Given the increasing burden on caregivers and their anxiety attached to caring for relatives with progressive disease as in the current and prior studies [3, 4], PC for GBM patients should include substantial, tangible support for caregivers. Neuropsychiatric symptoms in particular, with their effects on personality, self-confidence, relationships, and abilities, can be frightening and overwhelming for relatives as well as patients, potentially leading to feelings of helplessness [29–31]. Caregivers’ needs must be identified in order to be addressed, as patients’ well-being depends on caregivers’ well-being [32, 33]. The Zarit Burden Interview, originally developed to measure self-reported burden among caregivers of adults with dementia, may also be appropriate for use in caregivers of GBM patients [34], but a validated measure of caregiver quality of life specific to brain tumor scenarios would be a very useful tool since it is the patient–caregiver dyad that requires care—not the patient alone. In the current study, only three patients died at home. Substantial family caregiver support is necessary to fulfill most patients’ wishes to be cared for at home until death [32].
Improving GBM patient care
An essential way to improve home care for GBM patients is to improve in-home services as outpatient visits become impossible with disease progression. This is true for additional therapies like psychooncological treatment and physio-, occupational or speech therapy, all of special importance for GBM patients and rare in the current study population, as well as for in-home PC services. These might stabilize the home care setting for GBM patients [35], leading to fewer hospitalizations and increasing the likelihood of death at home [36]. Early, integrated PC, which has been found to improve quality of life for special cancer entities [37], may also help GBM patients. GBM patients are less likely to receive timely or PC services at all than other cancer patients [3, 10, 11, 38], which is consistent with the findings of the semi-structured interviews of the current study.
Limitations
For each assessment, the patient and/or caregiver decided whether it would be self, joint, or external. Our results may be limited due to lack of objective criteria for determining patients’ ability to participate in assessments. Due to increasing impairment, assessment was inconsistent over time (56 % self-assessment and 44 % joint/external assessment). A modeling approach was taken, i.e., incorporating the type of assessment as a co-factor, to guard against detection bias. In the presence of a statistically significant interaction with the type of assessment, specific relationships were investigated and interpreted. Due to the small sample size, we have to assume that this inferential approach comes with limited statistical power, albeit large enough to detect some distinct differences.
Conclusion
A prospective PC assessment of GBM patients from diagnosis to death is feasible if both self- and external assessments are allowed. The standard PC assessments identified the main burdens as psychosocial issues and increasing care dependency. The assessments should be amended with specific questions on neuropsychiatric symptoms and family caregiver burden as both are of striking importance and influence the well-being of both patients and caregivers.
Notes
The term “caregiver” is most widely used to refer to non-professionals assisting in the care of persons receiving palliative care. For our purposes here, this refers to relatives of the GBM patients caring for them, also sometimes called family caregivers.
Abbreviations
- HOPE:
-
Hospiz- und Palliativerhebung (Hospice and Palliative Care Evaluation)
- HOPE-SP-CL:
-
HOPE symptom and problem checklist
- POS:
-
Palliative Outcome Scale
- ECOG:
-
Eastern Cooperative Oncology Group
- PROM:
-
Patient-reported outcome measure
- PC:
-
Palliative care
- GBM:
-
Glioblastoma
- ADLs:
-
Activities of daily living
References
Ohka F, Natsume A, Wakabayashi T (2012) Current trends in targeted therapies for glioblastoma multiforme. Neurol Res Int 2012:878425. doi:10.1155/2012/878425
Walbert T, Khan M (2014) End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. J Neurooncol 117(2):217–224. doi:10.1007/s11060-014-1393-6
Ostgathe C, Gaertner J, Kotterba M, Klein S, Lindena G, Nauck F, Radbruch L, Voltz R, Hospice and Palliative Care Evaluation (HOPE) Working Group in Germany (2010) Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer 18(9):1157–1163. doi:10.1007/s00520-009-0735-y
Heese O, Vogeler E, Martens T, Schnell O, Tonn JC, Simon M, Schramm J, Krex D, Schackert G, Reithmeier T, Nikkah G, Sabel M, Steiger HJ, Schlegel U, Löffler M, Weller M, Westphal M, German Glioma Network (2013) End-of-life caregivers’ perception of medical and psychological support during the final weeks of glioma patients: a questionnaire-based survey. Neuro Oncol 15(9):1251–1256. doi:10.1093/neuonc/not089
Taphoorn MJB, Sizoo EM, Bottomley A (2010) Review on quality of life issues in patients with primary brain tumors. Oncologist 15(6):618–626. doi:10.1634/theoncologist.2009-0291
Bausewein C, Hau P, Borasio GD, Voltz R (2003) How do patients with primary brain tumours die? Palliat Med 17(6):558–559
Oberndorfer S, Lindeck-Pozza E, Lahrmann H, Struhal W, Hitzenberger P, Grisold W (2008) The end-of-life hospital setting in patients with glioblastoma. J Palliat Med 11(1):26–30. doi:10.1089/jpm.2007.0137
Pace A, Di Lorenzo C, Guariglia L, Jandolo B, Carapella CM, Pompili A (2009) End of life issues in brain tumor patients. J Neurooncol 91(1):39–43. doi:10.1007/s11060-008-9670-x
Sizoo EM, Braam LB, Postma TJ, Pasman HRW, Heimans JJ, Klein M, Reijneveld JC, Taphoorn MJB (2010) Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-Oncology 12(11):1162–1166. doi:10.1093/neuonc/nop045
Lin E, Rosenthal MA, Le BH, Eastman P (2012) Neuro-oncology and palliative care: a challenging interface. Neuro Oncol .14(4):iv3–7. doi:10.1093/neuonc/nos209
Faithfull S, Cook K, Lucas C (2005) Palliative care of patients with a primary malignant brain tumour: case review of service use and support provided. Palliat Med 19(7):545–550
HOPE Clara. HOPE project. [in German] Available from https://www.hope-clara.de. Accessed 16 April 2014
Radbruch L, Nauck F, Ostgathe C, Elsner F, Bausewein C, Fuchs M, Lindena G, Neuwöhner K, Schulenberg D (2003) What are the problems in palliative care? Results from a representative survey. Support Care Cancer 11(7):442–451
Stiel S, Pollok A, Elsner F, Lindena G, Ostgathe C, Nauck F, Radbruch L (2012) Validation of the symptom and problem checklist of the German Hospice and Palliative Care Evaluation (HOPE). J Pain Symptom Manag 43(3):593–605. doi:10.1016/j.jpainsymman.2011.04.021
Hearn J, Higginson IJ (1998) Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 8(4):219–227
Bausewein C, Fegg M, Radbruch L, Nauck F, von Mackensen S, Borasio GD, Higginson IJ (2005) Validation and clinical application of the German version of the Palliative care Outcome Scale. J Pain Symptom Manag 30(1):51–63
Brown CG, McGuire DB, Beck SL, Peterson DE, Mooney KH (2007) Visual graphical analysis: a technique to investigate symptom trajectories over time. Nurs Res 56:195–201
Mayring P (2004) Qualitative content analysis. In: Flick U, von Kardorff E, Steinke I (eds) A companion to qualitative research, 1st edn. Sage, London, pp 266–269
Glaser BG, Strauss AL (1998) Grounded theory. Strategien qualitativer Forschung, 1st edn. Hans Huber Verlag, Bern
Pope C, Mays N (eds) (2006) Qualitative research in health care, 3rd edn. Blackwell Publishing, Massachusetts
Sneeuw KC, Sprangers MA, Aaronson NK (2002) The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease. J Clin Epidemiol 55:1130–1143
Brown PD, Decker PA, Rummans TA, Clark MM, Frost MH, Ballmann KV, Arusell RM, Buckner JC (2008) A prospective study of quality of life in adults with newly diagnosed high grade glioma: comparison of patient and caregiver ratings of quality of life. Am J Clin Oncol 31(2):163–168
Higginson IJ, Gao W (2008) Caregiver assessment of patients with advanced cancer: concordance with patients, effect of burden and positivity. Health Qual Life Outcome 6:42. doi:10.1186/1477-7525-6-42
Jordhøy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M (1999) Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 13(4):299–310
Voltz R, Galushko M, Walisko J, Nauck F, Radbruch L, Ostgathe C (2010) End-of-life research on patients’ attitudes in Germany: a feasibility study. Support Care Cancer 18(3):317–320. doi:10.1007/s00520-009-0654-y
Gysels M, Shipman C, Higginson IJ (2008) Is the qualitative research interview an acceptable medium for research with palliative care patients and carers? BMC Med Ethics 9:7. doi:10.1186/1472-6939-9-7
Taphoorn MJ, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, Stupp R, Mirimanoff RO, van den Bent MJ, Bottomley A, EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups (2010) An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 46(6):1033–1040. doi:10.1016/j.ejca.2010.01.012
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314
Lucas MR (2010) Psychosocial implications for the patient with a high-grade glioma. J Neurosci Nurs 42(2):104–108. doi:10.1097/JNN.0b013e3181ce5a34
Cavers D, Hacking B, Erridge SE, Kendall M, Morris PG, Murray SA (2012) Social, psychological and existential well-being in patients with glioma and their caregivers: a qualitative study. CMAJ 184(7):E373–382. doi:10.1503/cmaj.111622
Flechl B, Ackerl M, Sax C, Oberndorfer S, Calabek B, Sizoo E, Reijneveld J, Crevenna R, Keilani M, Gaiger A, Dieckmann K, Preusser M, Taphoorn MJ, Marosi C (2013) The caregivers’ perspective on the end-of-life phase of glioblastoma patients. J Neurooncol 112(3):403–411. doi:10.1007/s11060-013-1069-7
Thomas K, Hudson P, Oldham L, Kelly B, Trauer T (2010) Meeting the needs of family carers: an evaluation of three home-based palliative care services in Australia. Palliat Med 24(2):183–191. doi:10.1177/0269216309351467
Stetz K, Brown M (1997) Taking care: caregiving to persons with cancer and AIDS. Cancer Nurs 20(1):12–22
Zarit SH, Reever KE, Bach-Peterson J (1980) Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 20(6):649–655
Sizoo EM, Roeline H, Pasman W, Dirven L, Marosi C, Grisold W, Stockhammer G, Egeter J, Grant R, Chang S, Heimans JJ, Deliens L, Reijneveld JC, Tabhoorn MJB (2014) The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer 22:847–857. doi:10.1007/s00520-013-2088-9
Pace A, Di Lorenzo C, Capon A, Villani V, Benincasa D, Guariglia L, Salvati M, Brogna C, Mantini V, Mastromattei A, Pompili A (2012) Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med 15(2):225–227. doi:10.1089/jpm.2011.0306
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363(8):733–742. doi:10.1056/NEJMoa1000678
Ostgathe C, Alt-Epping B, Golla H, Gaertner J, Lindena G, Radbruch L, Voltz R, Hospice and Palliative Care Evaluation (HOPE) Working Group (2011) Non-cancer patients in specialized palliative care in Germany: what are the problems? Palliat Med 25(2):148–152. doi:10.1177/0269216310385370
Acknowledgments
We would like to thank all of the study participants and their caregivers as well as the physicians involved for contacting patients.
Funding
The Center for Clinical Trials, University Hospital, Cologne, Germany, receives support for clinical studies from the Federal Ministry of Education and Research (BMBF01KN0706).
Conflict of interest
The authors declare that there is no conflict of interest. All authors have full control of all primary data and allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Additional information
Heidrun Golla and Maryam Ale Ahmad contributed equally to this work.
Rights and permissions
About this article
Cite this article
Golla, H., Ale Ahmad, M., Galushko, M. et al. Glioblastoma multiforme from diagnosis to death: a prospective, hospital-based, cohort, pilot feasibility study of patient reported symptoms and needs. Support Care Cancer 22, 3341–3352 (2014). https://doi.org/10.1007/s00520-014-2384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2384-z