Abstract
Purpose
Early detection and improvements in treatment have increased survival after colorectal cancer (CRC), but studies investigating the multidimensional nature of treatment-related symptoms are rare. The aim of this study was therefore to describe the prevalence, frequency, and severity of symptoms and the distress they cause during the early treatment of patients with CRC undergoing chemotherapy.
Methods
Consecutive outpatients were asked to rate their symptoms during cycle 2 or 3 of chemotherapy, using the Memorial Symptom Assessment Scale.
Results
A total of 104 patients, 58 men and 46 women, evaluated their symptoms of the preceding week at one point during the treatment. The mean number of symptoms was 10.3 (SD, 7.7; range, 0–32). Highly prevalent symptoms were numbness/tingling in the hands/feet (64 %), lack of energy (62 %), feeling drowsy (49 %), and nausea (45 %). Symptoms with the highest scores for frequency, severity, and distress were lack of energy followed by difficulty in sleeping and numbness in the hands/feet. Lack of energy was noted as occurring almost constantly by 26 % and was rated as being severe or very severe by 12 % and as quite distressing or very distressing by 15 %.
Conclusions
This study shows that patients with CRC receiving chemotherapy experience several distressing symptoms early in the treatment phase. In order to provide symptom control, oncology staff should consider evaluating the patient’s symptoms early during treatment and plan adequate measures to minimize the impact of treatment-induced toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is one of the most frequent malignancies worldwide and the second most common cause of cancer death for men and women [1]. Due to early detection and improvements in treatment, survival after 5 years is increasing [1, 2]. This means that the number of people living with and undergoing treatment for CRC is growing. The main treatment modalities for patients with CRC are surgery, radiotherapy, chemotherapy, and novel targeted therapies [2, 3]. Adjuvant chemotherapy after surgery, 5-fluorouracil (5-FU) in combination with folinic acid (leucovorin) and oxaliplatin, can reduce the risk of relapse with about 25 % of patients [2]. The introduction of novel target therapies such as bevacizumab and cetuximab has further increased the treatment options [2]. Depending not only on the disease itself but also the type of treatment, the patient can experience multiple symptoms. Well known side effects of chemotherapy combined with targeted therapies are peripheral neuropathy, fatigue, diarrhea, skin-related toxicities, nausea, and vomiting [4]. Side effects can lead to treatment dose limitation [5] and may affect patients’ ability to cope with everyday life [6]. It has been suggested that one major reason for inadequate symptom control is lack of effective symptom assessment [7]. Since symptoms are multidimensional experiences, there is a growing consensus in health care literature that symptom assessment should cover the underlying components of frequency, duration, severity or intensity, and distress [6].
Patients’ multidimensional experience of symptoms has previously been explored in earlier studies involving cancer patients [8–11], but none of these studies have included a homogenous group of patients with CRC undergoing chemotherapy. Earlier studies investigating symptoms and symptom management in patients with CRC have primarily focused on one-dimensional symptom scales, assessing prevalence, frequency, or severity for one or a few symptoms [8, 12, 13]. Tofthagen and McMillan, for example, focused on hand–foot syndrome in their study on patients with CRC treated with oxaliplatin and described neuropathy as often interfering with daily life [14]. Other symptoms that often bother patients with CRC were not explored in their study. Lynch et al. [15], studying patients with CRC 6 and 12 months after diagnosis, described the prevalence of psychological distress, but physical symptoms were not studied. Early systematic assessment and management of chemotherapy-related symptoms may improve symptom outcomes [16]. To our knowledge, no study has previously focused on describing the multidimensional nature of symptoms in a homogenous group of patients with CRC undergoing chemotherapy early in their treatment phase. Therefore, the purpose of this study was to describe the prevalence, frequency, and severity of symptoms and the distress they cause during the early treatment of patients with CRC undergoing chemotherapy.
Methods
In a cross-sectional study, patients with CRC were consecutively recruited from a university hospital and a county hospital in southeast Sweden between September 2008 and December 2009.
Participants
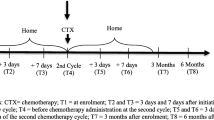
Eligibility criteria were adult patients (≥18 years) with a CRC diagnosis, admitted for chemotherapy treatment at one of the two involved departments. Patients treated with capecitabine-based chemotherapy were included at cycle 2, and patients treated with oxaliplatin were included at cycle 3. Exclusion criteria were patients with concomitant radiotherapy, patients treated with chemotherapy for the preceding 12 months, patients assessed by a physician as unable to participate and perform the assessments, or patients not having the ability to speak, write, or understand Swedish.
A total of 376 patients were treated during the recruitment period. Of these, 220 did not meet the inclusion criteria and 12 were missed due to logistic errors. The remaining 144 patients were given oral and written information about the aim of the study and a request for participation by their physician, at their initial treatment planning visit. One week later, the patients were contacted by a research nurse via telephone to provide further information and obtain informed consent. In total, 114 patients agreed to participate and signed informed consent forms, while 30 declined participation. During the survey, ten patients were withdrawn from the study (four because they had treatment changes, one died, one discontinued treatment due to complications, two withdrew participation without stating a reason, and two patients did not send in the questionnaire). In total, 104 (91 %) completed the survey, 58 men and 46 women. A majority of the patients had undergone surgery before chemotherapy treatment. The study was performed in accordance with the Declaration of Helsinki and Swedish legislation of noninvasive studies [17] and was approved by the Regional Ethical Review Board (M4-08 2008-02-27).
Measurement
Symptom assessment was performed, using the Memorial Symptom Assessment Scale (MSAS) [18, 19] which is a well-validated and reliable self-report instrument, also in patients with CRC [8, 18, 19]. The MSAS measures the prevalence, frequency, severity, and distress of 25 physical symptoms and seven psychological symptoms. In addition to the 32 predefined symptoms listed in the questionnaire, participants could also report other occurring symptoms.
For eight symptoms, only severity and distress are evaluated in the MSAS. The patients were asked to record the symptoms as present or absent during the past 7 days. If present, the frequency of a symptom was rated on a four-point scale ranging from 1 = rarely to 4 = almost constantly and severity from 1 = slightly to 4 = very severe. Distress was rated on a five-point scale ranging from 0 = not at all to 4 = very much [18].
Translation of the MSAS to Swedish and back translation to English was conducted in three steps [20, 21]. First, all items were translated into Swedish by a native translator. This version was checked by the authors and was then independently back translated into English by a native-speaking translator. The authors compared the back translation with the original version, and no cultural dilemmas could be found. After concluding these steps, 40 patients evaluated the ease of use, understanding of items, and respondent burden. None of these patients were later included in the present study. Minimal revisions such as adapting words to the everyday Swedish language were required before implementation in the main study.
Sociodemographic characteristics including age, sex, level of education, social status (living alone, with partner, children at home), employment, and clinical characteristics including comorbidities were obtained using a study-specific questionnaire, and chemotherapy treatment was obtained by chart review.
Procedure
After obtaining written informed consent, the research nurse handed out a survey package including the MSAS to the patients at different times, depending on what chemotherapy regime they were receiving. Those treated with capecitabine received the questionnaires at chemotherapy cycle 2, and those treated with an oxaliplatin-based regime received their questionnaires at chemotherapy cycle 3. The differences in time frame were to ensure that the patients had experienced a similar volume intensity of chemotherapy at the time of data collection. The questionnaire was to be answered by the patients, in their own homes, at the end of the following 1-week “rest period” of treatment, and was to be sent to the researchers in a prepaid envelope.
Data analysis
MSAS scores in this study were calculated as described by Portenoy et al. [18]. To ease calculation, Portenoy recommends converting the 5-graded distress scale (0–4) as follows: 0 = 0.8, 1 = 1.6, 2 = 2.4, 3 = 3.2, and 4 = 4 [18]. The initial step calculates a score for each symptom. If a symptom is not experienced, the score for that symptom is 0. If a symptom is experienced, the score for that symptom is determined as the average of the scores on frequency, severity, and distress (MSAS score). This initial calculation forms the basis for further calculations of three MSAS subscales (described below). However, for the purpose of this study, we focused less on the subscales and chose to illustrate the patients’ detailed descriptions instead by providing percentages of frequency, severity, and distress for each of the studied symptoms. The percentages of patients scoring symptom prevalence were calculated as well as the proportion of those who did not have the symptoms or did not answer the questions. We also calculated the percentages of patients scoring symptom frequency as “frequently or almost constantly” to high scores (≥3) and patients scoring “rarely or occasionally” to low scores (≤2). Patients scoring symptom severity as “severe or very severe” were classified as high scores (≥3) and those rating severity as “slightly/moderate” to low scores (≤2). Patients scoring symptom distress as “quite a bit or very much” were allocated to high scores (≥3) and those scoring “not at all or a little bit or somewhat” were allocated low scores (≤2).
Calculations of the three MSAS subscales were performed: the Physical Symptom Subscale (MSAS-PHYS), the Psychological Symptom Subscale (MSAS-PSYCH), and the Global Distress Index (MSAS-GDI). MSAS-PHYS is the average score for lack of appetite, lack of energy, pain, feeling drowsy, constipation, dry mouth, nausea, vomiting, change in taste, weight loss, feeling bloated, and dizziness. MSAS-PSYCH is the average score for feeling sad, worrying, feeling irritable, feeling nervous, difficulty in sleeping, and difficulty in concentrating. MSAS-GDI is the average score for the frequency of feeling sad, worrying, and feeling irritable. The distress scores are for lack of appetite, lack of energy, pain, feeling drowsy, constipation, and dry mouth.
The total MSAS score (TMSAS) was calculated as the average of all patients’ MSAS scores, determined by the number of symptoms experienced and the various ratings of each symptom. Both the MSAS-GDI and TMSAS are considered to be measures of overall symptom distress. Higher values indicate greater distress [18, 22].
Data analysis was performed using SPSS software version 15, and descriptive statistics were used to describe the patients’ sociodemographics and clinical characteristics.
Results
Of the included 104 patients, 58 (56 %) were males and 46 (44 %) were females, ranging from 37–85 years of age. All were outpatients at the time of the survey. The patients’ sociodemographic and clinical characteristics are presented in Table 1.
Two thirds of the patients were partnered or lived with the family, and 50 % were retired. Nearly two thirds (68 %) reported having one or more diseases apart from CRC. The four most prevalent other types of disease were cardiovascular (42 %) followed by intestinal (10 %), diabetes (9 %), and pulmonary (8 %). A majority of the patients were treated either with capecitabine as a single drug (36 %) or with oxaliplatin-based combinations (50 %). Nine percent received chemotherapy combined with a monoclonal antibody. Treatment intentions were curative (3 %), adjuvant (47 %), neoadjuvant (20 %), or palliative (30 %).
Symptom prevalence
The mean of symptom prevalence was 10.3 (range, 0–32; SD, 7.7) symptoms. The most prevalent physical symptoms, experienced by more than 40 % of the participants, were numbness/tingling in the hands/feet (64 %), followed by lack of energy (62 %), feeling drowsy (49 %), nausea (45 %), shortness of breath (43 %), and dry mouth (42 %) (Fig. 1).
Difficulty sleeping (46 %) and worrying (44 %) were the two most common psychological symptoms. The least common symptoms experienced were hair loss (14 %) and “I don’t look like myself” (12 %). In addition to the 32 predefined symptoms in MSAS, 23 % of the patients reported other symptoms; dry nose with nose bleeding was experienced by 5 %, dry skin by 4 %, mouth problems by 4 %, problems when walking by 3 %, eye problems by 3 %, clumsiness by 2 %, hoarseness by 2 %, and increased hair growth 1 %. Five (5 %) patients reported not having any symptoms at all.
Symptom frequency, severity, and distress
The proportion of patients reporting high (≥3) or low (≤2) scores for frequency, severity, and distress of symptoms, as well as missing values, are presented in Fig. 2. For almost all symptoms, patients reported higher scores for frequency than for severity or distress. For example, 16 (15 %) of the patients scored high (≥3) (frequently or almost constantly) on frequency of “problems with sexual interest or activity,” while 8 (8 %) of them scored high (severe or very severe) on the severity of that problem. Six (6 %) of the patients scored high (quite a bit or very much) on the distress dimension of the problem. Between 1 % (mouth sores) and 20 % (feeling drowsy) of the patients reporting prevalence of a symptom did not rate the frequency, severity, or distress of the same symptom. The distress dimension was the item with the most missing data (5–20 %), with 5 % missing data for “I don’t look like myself” and “hair loss and 20 % missing data for “feeling drowsy.”
Percentages of patients (n = 104) scoring symptom frequency (F) as “frequently (3)/almost constantly (4)” = ≥3 or “rarely (1)/occasionally (2)” = ≤2, symptom severity (S) as “severe (3)/very severe (4)” = ≥3 or “slightly (1)/moderate (2)” = ≤2, and symptom distress (D) as “quite a bit (3)/very much (4)” = ≥3 or “not at all (0)/a little bit (1)/somewhat (2)” = ≤2 with MSAS. Percentages of patients not having the symptoms as well as those not answering the questions are also shown. A patient can score more than one symptom
The ten most prevalent symptoms and the percentage of patients reporting high (≥3) scores for frequency, severity, and distress on each of these symptoms are presented in Fig. 3. Symptoms with the highest scores for all the three dimensions (frequency, severity, distress) were lack of energy followed by difficulty sleeping and numbness in the hands/feet. They were mentioned as occurring almost constantly by 11, 4, and 7 %, respectively, as very severe by 2, 2, and 0 %, respectively, and as very distressing by 2, 2, and 2 %, respectively.
The ten most prevalent symptoms based on answers by 104 patients scoring symptoms with MSAS. A patient can score more than one symptom. Percentages of patients reporting high scores (≥3) on frequency (F), severity (S), and distress (D) as frequently/almost constantly, severe/very severe, and quite a bit/very much, respectively
MSAS subscales
MSAS subscale scores were calculated, as described by Portenoy et al. [18], and explained in the analysis section, only for the 63 patients who completed scoring of all symptom dimensions of the items. The means for the MSAS-PHYS and MSAS-PSYCH were 0.58 (range, 0–2.64) and 0.61 (range, 0–3.07), respectively. The means for MSAS-GDI and TMSAS were 0.68 (range, 0–2.96) and 0.54 (range, 0–1.88), respectively.
Discussion
The results from this study suggest that patients treated with chemotherapy for CRC early in their treatment phase can experience multiple symptoms. The patients reported a mean of ten symptoms per individual, and the most prevalent symptoms were numbness/tingling in the hands and feet, lack of energy, feeling drowsy, and difficulty in sleeping. Of those, lack of energy was the most distressing to patients. Although we found a low proportion of patients reporting high distress from different symptoms, the results suggest that regular symptom assessment at the beginning of chemotherapy treatment is important for appropriate care measures since many of the symptoms can increase during the treatment [5].
The mean of symptoms per patient found in this study is in line with other findings on patients with cancer [23]. Spichiger et al. showed a significant increase in number of symptoms experienced from 9.8 at the start of chemotherapy to 14.4 by cycle 3 [24]. Absolute freedom from symptoms may not be realistic in patients with CRC during chemotherapy but the high prevalence in the early treatment phase raises the importance of longitudinal studies investigating the impact of symptoms during the entire treatment phase.
Numbness/tingling in the hands and feet, often caused by chemotherapy used in CRC treatment, was mentioned by 64 %, which is surprising since the patients had only received two or three cycles of chemotherapy. This is in accordance with results from Rosati et al., who found early acute neuropathy effects in 12 % of 21 patients with CRC during their first cycle with oxaliplatin [25]. Although the patient number was small, they saw an escalation of peripheral neuropathy over time. An escalation over time was also shown by Chou et al. [11], who studied hand/feet symptoms in cancer patients receiving chemotherapy, including patients with CRC. Similar results were found by Deshields et al., investigating patients with CRC 6–8 months after diagnosis [9]. These findings thus support the view that patients should be informed and educated about neurotoxicity so they can assess early changes, and it is also important that they know how to report these changes to the healthcare personnel responsible for their care [26].
The second most frequent symptom in our study was lack of energy, though when looking at the proportion of patients scoring the symptom as present frequently or almost constantly, lack of energy was ranked top. The same applies to the dimensions of severity and distress of lack of energy. This is in accordance with the study by Lam et al. [27], although the heterogeneity in treatment of their sample of patients with CRC was large since it also included patients with no active treatment, patients on radiation therapy, or on chemoradiation therapy. A recent study of 558 patients with varied cancer diagnoses also reported lack of energy as one of the top symptoms for all types of cancer, and more than 75 % of patients with CRC experienced lack of energy [9]. It is noteworthy that these patients were at various stages of treatment. A study by Cheng et al. [10] supported the high prevalence of lack of energy among patients with CRC 12 months after treatment. The prevalence in their study (44 %) was a little lower than our findings (60 %), which may indicate that the symptoms decrease after treatment. Lack of energy is a concept close to cancer-related fatigue, which has been reported as persistent and distressing [28]. It has been documented to be highly prevalent, underreported, and untreated in cancer patients [29], and patients with CRC report fatigue as a primary concern [30]. Taking into consideration that the patients in our study were only at the beginning of their chemotherapy, our results indicate the importance of informing patients about fatigue at the start of the treatment and of increasing efforts to find strategies to reduce fatigue.
In this study, we found that sleeping difficulty was one of the most prevalent psychological symptoms and was experienced by nearly half of the patients. It had the second highest number of patients, when analyzing those with the most severe symptoms. Several studies including patients with cancer have shown a similar or higher frequency of sleeping problems [11, 24, 31]. The combination of fatigue and sleep disturbances during adjuvant therapy for patients with CRC has been shown to cause a risk of excessive time spent in bed and low activity, which may lead to worsening of physical functioning and loss of independence [32].
Problems with sexual interest or activity were mentioned by one third of the patients in the study. This is in line with other findings [9, 23, 27] but higher than others reported [10, 31, 33]. Some studies did not report sexual problems since the patients had not answered the question [24, 34] or because the researchers considered the question to be too sensitive for the population [35]. Questions about problems with sexual interest or activity may seem intrusive. Despite a high proportion of answers in our study, this question had the highest number of missing answers for frequency, severity, and distress. Cheng et al. [10] point out that there is a risk of patients underreporting this symptom due to culture and taboo. A study by Di Fabio and Koller [36] points out the correlation between sexual dysfunction and lower quality of life and suggests that more efforts should be made in clinical practice to investigate patients’ sexual issues. Cancer patients, especially those treated with chemotherapy, have reported needs for information and support in regard to sexual issues [37, 38]. It thus seems important to discuss the sexual consequences of disease and treatment with patients.
As a complement to the detailed descriptions of frequency, severity, and distress, we also calculated MSAS subscale scores. We found the results in accordance with studies on similar patient groups [27, 31], for example, a study by Lam et al. [27] investigating Chinese patients with CRC. Their results for the MSAS-PHYS and MSAS-PSYCH were a mean of 0.57 (range, 0–3.27) and 0.87 (range, 0–3.83), respectively. The means for MSAS-GDI and TMSAS were 0.82 (range, 0–3.18) and 0.57 (range, 0–3.83), respectively. Our results on the MSAS subscales were however lower than in studies with patients having a more advanced disease or other cancer diagnoses [31, 39]. One example is the study by Chang et al. [31], where the means for the MSAS-PHYS and MSAS-PSYCH scales in a group of patients with diverse tumor types with metastatic disease were 1.16 (SD, 0.75) and 0.91 (SD, 0.87), respectively. The means for MSAS-GDI and TMSAS were 1.29 (SD, 0.80) and 0.85 (SD, 0.52), respectively.
A study on patients with breast cancer reported higher symptom scores on MSAS as significantly associated with decreased health and functioning [12]. For research purposes, the MSAS subscale scores may be useful but we recommend considering the raw scores for symptom frequency, severity, and distress when healthcare professionals need to communicate with patients about their symptoms.
Strength and limitations
MSAS was originally developed for use in cancer patients by Portenoy et al. [27]. Since then MSAS has been translated into several languages [27, 34, 39, 40] and has also been used for patients with different diseases [41, 42].
One strength of this study is the presentation of detailed information on all the dimensions of all symptoms, which is in contrast to studies only presenting data evaluated on MSAS subscores [31], mean of symptom severity or distress [8, 24] or the mean score of frequency, severity, and distress for the ten most prevalent symptoms [9]. The advantage of presenting all dimensions separately is that it gives a detailed picture of the symptom profile and complexity of symptoms during chemotherapy for patients with CRC. Although Portenoy recommends the use of subscales as a brief and easy global illustration of symptoms, we recommend the use of detailed descriptions of the symptom dimensions for a full overview. The use of mean scores on ordinal data may be regarded as statistically unorthodox. However, this is done on the subscales due to Portenoy’s instructions when analyzing MSAS.
Although the study sample was homogeneous, including a relatively large sample of patients in the same situation, this study has some limitations that the authors wish to acknowledge. Due to the study design, the data is limited to one data point. Future studies need to consider the use of a longitudinal design to identify symptom patterns that might change over time.
MSAS is described as one of the few questionnaires covering the most prevalent symptoms and as a comprehensive questionnaire to evaluate symptom multidimensionally, but some patients can find it bothersome to fill in the form [43]. Although the patients received detailed instructions from our research nurses, the complexity of the MSAS may have led to high levels of missing data in a number of cases, especially with regard to the dimension of distress. A consequence of the incomplete data is a risk that the multidimensional description of symptoms has still not fully described. Problems with uncompleted forms are also reported by Kris and Dodd [35]. This highlights the importance of giving detailed instructions when using MSAS and of considering using MSAS as an interview form, as Lam et al. [27] exemplified, when studying fragile or fatigued patients.
Conclusion
Our study shows that even at an early stage in the treatment phase, patients with CRC receiving chemotherapy can experience multiple distressing symptoms. The most frequent physical symptoms were numbness/tingling in the hands/feet and lack of energy, whereas the most frequent psychological symptoms were difficulty sleeping and worrying. All four symptoms are complex and may not be easily treated. Although the distress dimension of the symptom scoring generally was low, this may change later in the treatment phase. Prospective studies covering a multidimensional assessment of patients’ symptoms throughout the entire treatment phase are therefore suggested to increase our knowledge of the interactions between symptom frequency, severity, and distress among patients with CRC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, de Braud F, Wils J (2010) Colon cancer. Crit Rev Oncol Hematol 74(2):106–133. doi:10.1016/j.critrevonc.2010.01.010
Simpson J, Scholefield JH (2008) Treatment of colorectal cancer: surgery, chemotherapy and radiotherapy. Surg (Oxford) 26(8):329–333
Grenon NN, Chan J (2009) Managing toxicities associated with colorectal cancer chemotherapy and targeted therapy: a new guide for nurses. Clin J Oncol Nurs 13(3):285–296
Holt K (2011) Common side effects and interactions of colorectal cancer therapeutic agents. J Pract Nurs 61(1):7–20
Myers JS (2009) A comparison of the theory of unpleasant symptoms and the conceptual model of chemotherapy-related changes in cognitive function. Oncol Nurs Forum 36(1):E1–E10. doi:10.1188/09.ONF.E1-E10
Naughton M, Homsi J (2002) Symptom assessment in cancer patients. Curr Oncol Rep 4(3):256–263
Stark LL, Tofthagen C, Visovsky C, McMillan SC (2012) The symptom experience of patients with cancer. J Hospice Palliat Nurs 14(1):61–70. doi:10.1097/NJH.0b013e318236de5c
Deshields TL, Potter P, Olsen S, Liu J, Dye L (2011) Documenting the symptom experience of cancer patients. J Support Oncol 9(6):216–223. doi:10.1016/j.suponc.2011.06.003
Cheng KKF, Thompson DR, Ling MW, Chan CWH (2005) Measuring symptom prevalence, severity and distress of cancer survivors. Clin Eff Nurs 9(3–4):154–160
Chou F, Dodd M, Abrams D, Padilla G (2007) Symptoms, self-care, and quality of life of Chinese American patients with cancer. Oncol Nurs Forum 34(6):1162–1167. doi:10.1188/07.onf.1162-1167
Abu-Saad Huijer H, Abboud S (2012) Health-related quality of life among breast cancer patients in Lebanon. Eur J Oncol Nurs. doi:10.1016/j.ejon.2011.11.003
Kamil M, Haron M, Yosuff N, Khalid I, Azman N (2010) High frequency of hand foot syndrome with capecitabine. J Coll Physicians Surg Pak 20(6):421–422
Tofthagen C, McMillan S (2009) Peripheral neuropathy in colon cancer patients receiving oxaliplatin. Oncol Nurs Forum 36(3):70
Lynch BM, Steginga SK, Hawkes AL, Pakenham KI, Dunn J (2008) Describing and predicting psychological distress after colorectal cancer. Cancer 112(6):1363–1370. doi:10.1002/cncr.23300
Kearney N, Miller M, Maguire R, Dolan S, MacDonald R, McLeod J, Maher L, Sinclair L, Norrie J, Wengstrom Y (2008) WISECARE+: results of a European study of a nursing intervention for the management of chemotherapy-related symptoms. Eur J Oncol Nurs 12(5):443–448
World Medical Association (2008) Medical Association Declaration of Helsinki – ethical principles for medical research involving human patients. http://www.wma.net/en/30publications/10policies/b3/. Accessed 1303 2013
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L et al (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30A(9):1326–1336
Chang VT, Thaler HT, Polyak TA, Kornblith AB, Lepore JM, Portenoy RK (1998) Quality of life and survival: the role of multidimensional symptom assessment. Cancer 83(1):173–179. doi:10.1002/(SICI)1097-0142(19980701)83:1<173::AID-CNCR23>3.0.CO;2-T
Brislin RW (1970) Back-translation for cross-cultural research. J Cross-Cultur Psychol 1:185–216
Acquadro C, Conway K, Giroudet C, Mear I (2012) Linguistic validation manual for health outcome assessments. MAPI Institute, Lyon
Lobchuk MM, Degner LF, Chateau D, Hewitt D (2006) Promoting enhanced patient and family caregiver congruence on lung cancer symptom experiences. Oncol Nurs Forum 33(2):273–282. doi:10.1188/06.onf.273-282
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Coyle N, Smart-Curley T, Kemeny N, Norton L, Hoskins W et al (1994) Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 3(3):183–189
Spichiger E, Muller-Frohlich C, Denhaerynck K, Stoll H, Hantikainen V, Dodd M (2011) Prevalence of symptoms, with a focus on fatigue, and changes of symptoms over three months in outpatients receiving cancer chemotherapy. Swiss Med Wkly 141:w13303. doi:10.4414/smw.2011.13303
Rosati G, Rossi A, Tucci A, Pizza C, Manzione L (2001) Phase I study of a weekly schedule of oxaliplatin, high-dose leucovorin, and infusional fluorouracil in pretreated patients with advanced colorectal cancer. Ann Oncol 12(5):669–674
Berg D (2003) Oxaliplatin: a novel platinum analog with activity in colorectal cancer. Oncol Nurs Forum 30(6):957–966
Lam WWT, Law CC, Fu YT, Wong KH, Chang VT, Fielding R (2008) New insights in symptom assessment: the Chinese versions of the Memorial Symptom Assessment Scale Short Form (MSAS-SF) and the Condensed MSAS (CMSAS). J Pain Symptom Manag 36(6):584–595
National Comprensive Cancer Network (2012) NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue. http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed 10 October 2012
Scott JA, Lasch KE, Barsevick AM, Piault-Louis E (2011) Patients’ experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncol Nurs Forum 38(3):E191–203. doi:10.1188/11.onf.e191-e203
Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D (2008) Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw 6(5):448–455
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000) The memorial symptom assessment scale short form (MSAS-SF). Cancer 89(5):1162–1171
Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM (2010) Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum 37(6):E359–369. doi:10.1188/10.onf.e359-e369
Chaiviboontham S, Viwatwongkasem C, Hanucharurnkul S, McCorkle R (2011) Symptom clusters in Thais with advanced cancer. Pac Rim Int J Nurs Res 15(4):265–277
Spichiger E, Müller-Fröhlich C, Denhaerynck K, Stoll H, Hantikainen V, Dodd M (2011) Symptom prevalence and changes of symptoms over ten days in hospitalized patients with advanced cancer: a descriptive study. Eur J Oncol Nurs 15(2):95–102. doi:10.1016/j.ejon.2010.06.005
Kris AE, Dodd MJ (2004) Symptom experience of adult hospitalized medical-surgical patients. J Pain Symptom Manag 28(5):451–459
Di Fabio F, Koller M, Nascimbeni R, Talarico C, Salerni B (2008) Long-term outcome after colorectal cancer resection. Patients’ self-reported quality of life, sexual dysfunction and surgeons’ awareness of patients’ needs. Tumori 94(1):30–35
Sanson-Fisher R, Girgis A, Boyes A, Bonevski B, Burton L, Cook P (2000) The unmet supportive care needs of patients with cancer. Supportive Care Review Group. Cancer 88(1):226–237
Rasmusson EM, Plantin L, Elmerstig E (2013) ‘Did they think I would understand all that on my own?’ A questionnaire study about sexuality with Swedish cancer patients. Eur J Cancer Care 22(3):361–369. doi:10.1111/ecc.12039
Akin S, Can G, Aydiner A, Ozdilli K, Durna Z (2010) Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur J Oncol Nurs 14(5):400–409. doi:10.1016/j.ejon.2010.01.003
Sumdaengrit B, Hanucharurnkul S, Dodd MJ, Wilailak S, Vorapongsathorn T, Pongthavornkamol K (2010) Symptom experience and self-care among Thai women with cervical cancer. Pacific Rim Int J Nurs Res 14(3):203–218
Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK (2009) Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manag 38(1):115–123
Zambroski CH, Moser DK, Bhat G, Ziegler C (2005) Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs 4(3):198–206. doi:10.1016/j.ejcnurse.2005.03.010
Strömgren AS, Groenvold M, Pedersen L, Olsen AK, Sjogren P (2002) Symptomatology of cancer patients in palliative care: content validation of self-assessment questionnaires against medical records. Eur J Cancer 38(6):788–794. doi:10.1016/s0959-8049(01)00470-1
Acknowledgments
We thank the research nurses Monica Rösliden and Cecilia Blad for monitoring the data collection in the survey. We also want to thank members of the research team who planned the large project that this study is part of Hans Starkhammar, Susanne Borén, Viktoria Markusson, and Ursula Falkmer. This study was supported by the Medical Research Council of Southeast Sweden and the Division of Nursing Science at the Department of Medical and Health Sciences, Faculty of Health Sciences, Linköping University.
Clinical implications
The results suggest that regular symptom assessment is important even at the beginning of chemotherapy treatment in order to be able to provide effective treatment for symptoms as soon as they occur. In addition, it may be important to include dimensions such as severity and distress as a complement to assessing the frequency of a symptom.
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettersson, G., Berterö, C., Unosson, M. et al. Symptom prevalence, frequency, severity, and distress during chemotherapy for patients with colorectal cancer. Support Care Cancer 22, 1171–1179 (2014). https://doi.org/10.1007/s00520-013-2069-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-2069-z