Abstract
Objectives
Neurokinin-1 receptor antagonists (NK1 RAs) are commonly coadministered with a 5-HT3 RA such as palonosetron to prevent nausea and vomiting induced by chemotherapy. Netupitant, a new highly selective NK1 RA, is both a substrate for and a moderate inhibitor of CYP3A4. Three studies were designed to evaluate the potential drug–drug interaction of netupitant with palonosetron and of the fixed dose combination of netupitant and palonosetron, NEPA, with an inhibitor (ketoconazole), an inducer (rifampicin) and a substrate (oral contraceptives) of CYP3A4.
Methods
Study 1 was a three-way crossover in 18 healthy subjects receiving netupitant alone, palonosetron alone, and the combination of both antiemetics. Studies 2 and 3 were two-way crossover trials where healthy subjects received NEPA (the fixed dose combination of netupitant and palonosetron). In study 2, 36 subjects received NEPA alone (day 1) and in combination with ketoconazole or rifampicin. In study 3, 24 healthy women received ethinylestradiol/levonorgestrel alone or in combination with NEPA (day 1).
Results
There were no significant pharmacokinetic interactions between netupitant and palonosetron. Ketoconazole increased netupitant area under curve (AUC) by 140 % and C max by 25 %. Rifampicin decreased netupitant AUC by 83 % and C max by 62 %. NEPA did not significantly affect exposure to ethinylestradiol, while systemic exposure to levonorgestrel increased by 40 %, but this was not considered clinically relevant.
Conclusions
There were no clinically relevant interactions between netupitant and palonosetron, or between NEPA and oral contraceptives. The coadministration of NEPA with inhibitors or inducers of CYP3A4 may require dose adjustments. Treatments were well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) represents one of the most feared side effects of chemotherapy [1,2]. Several medical associations, such as the Multinational Association of Supportive Care in Cancer, developed international antiemetic guidelines aimed at supporting oncologists and hematologists in the choice of the most effective antiemetic prophylaxis. To date, the combination of an NK1 receptor antagonist (RA) with a 5-HT3 RA and a corticosteroid is the recommended antiemetic regimen for patients receiving highly emetogenic chemotherapy [3–5]. The 5-HT3 RA and NK1 RA are thought to target two distinct pathways involved in the emetic response during the acute (day 1 after chemotherapy) and delayed (from day 2 to 5 after chemotherapy) phases of CINV, respectively [6]. Although not completely understood, a cross-talk between the 5-HT3 and the NK1 receptor pathways exist [7], and palonosetron, a clinically superior [8–11] and pharmacologically distinct 5-HT3 RA, uniquely demonstrates the ability to inhibit it [12–14].
Netupitant (2-(3,5-bis-trifluromethyl-phenyl)-N-methyl-N-[6-(4-methyl-piperazin-1-yl)-4-o-tolyl-pyridin-3-yl]-isobutyramide) is a new, highly selective antagonist of the human NK1 receptor [15]. Netupitant is being developed as an oral fixed dose combination with palonosetron [NEPA (300 mg netupitant plus 0.5 mg palonosetron)] to provide a unique single-dose treatment that targets the two major emetic pathways. A synergistic effect between netupitant and palonosetron has recently been described by Stathis et al. [14], suggesting the potential for an improved efficacy of this fixed dose combination in clinical practice.
Both palonosetron and netupitant are eliminated by oxidative processes through the liver, but each of them is primarily metabolized by different cytochrome P450 isoenzymes. In particular, netupitant is mainly metabolized through the CYP3A4, while palonosetron is metabolized through the CYP2D6, with minor contributions from the CYP1A2 and CYP3A4 systems.
The in vitro inhibition and induction potential of netupitant on the CYP1A2, CYP2C19, CYP2D6, and CYP2C9 systems was evaluated in previous studies, and, based on these results, no significant metabolic drug–drug interactions in human are expected for compounds metabolized through these isoenzymes ([16] + data on file). An inhibitory effect of netupitant on the CYP3A4 was shown in vitro, and a recently published phase I clinical study using midazolam and erythromycin as CYP3A4 substrate probe demonstrated that netupitant is a moderate CYP3A4 inhibitor [17]. At clinical doses, palonosetron has no induction or inhibition potential for any of these isoenzymes [18].
The potential for drug interactions of netupitant or NEPA has been assessed in three different studies. In the first study, an evaluation of any effects of palonosetron on netupitant pharmacokinetics (PK) and of netupitant on palonosetron PK was studied. The other two studies were designed to evaluate the drug–drug interaction potential of NEPA with agents that could be coadministered in clinical practice. As netupitant is a substrate of the CYP3A4, NEPA was studied with the prototypical CYP3A4 strong inhibitor, ketoconazole, and an inducer, rifampicin, in order to assess the magnitude of this interaction. In the third study, the impact of the moderate inhibitory activity of NEPA was evaluated on the PK of ethinylestradiol/levonorgestrel oral contraceptive, a substrate of CYP3A4 that could be coadministered in clinical practice.
Materials and methods
Study population
In all studies, adult healthy volunteers who signed the informed consent were eligible for inclusion. Subjects who were receiving any concomitant medications that could potentially interact with the study drugs or had known contraindication to any 5-HT3 or NK1 receptor antagonists were excluded. The netupitant/palonosetron drug–drug interaction (DDI) study enrolled nine men and nine women (age range, 18–43 years), while the NEPA/ketoconazole-rifampicin DDI study enrolled 21 men and 15 women (age range, 32–55 years). For the NEPA/oral contraceptive DDI study, 24 healthy women aged 18–40 years inclusive were eligible, provided they had not been using any hormonal contraception for 3 months prior to study entry and were using a highly effective means of birth control (Table 1).
The studies were conducted at Quintiles Hermelinen, Sweden (netupitant/palonosetron DDI study) and at CRS Clinical Research Services Mannheim GmbH, Mannheim, Germany (NEPA/ketoconazole-rifampicin DDI study and NEPA/oral contraceptive DDI study).
The study protocols and informed consent documents were reviewed and approved by an independent ethics committee. Subjects provided written informed consent prior to participation in any study procedures. All investigators and site personnel were required to follow the Good Clinical Practice, International Conference on Harmonization, and Declaration of Helsinki principles and local laws and regulations.
Study Designs and Assessments
All studies were randomized, open-label, crossover designs and are summarized in Table 2. Eligible subjects fasted for at least 10 h before dosing in each period. Assigned doses of study drug were administered, and serial blood samples were obtained for PK analysis of netupitant and its metabolites (M1, M2, and M3), palonosetron and its metabolites (M4 and M9), ethinylestradiol, and levonorgestrel. Appropriate washout periods separated the treatments. Safety was evaluated by reviewing clinical laboratory results, electrocardiogram results, vital signs, physical examination findings, and the occurrence of any adverse events (AEs). Concomitant medications were not permitted during any study, with the exception of medications to treat adverse events.
Bioanalytical methods
In the netupitant/palonosetron DDI study, netupitant and its metabolites and palonosetron and its metabolites were measured in plasma using a validated liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) procedure in the Department of Bioanalytics of CRS, Mannheim GmbH, Grünstadt.
In the NEPA/ketoconazole–rifampicin DDI study, the analysis of netupitant and its metabolites and palonosetron was performed in plasma through a validated LC/MS/MS method.
In the oral contraceptive DDI study, the analysis of ethinylestradiol and levonorgestrel in plasma was performed through a validated internally standardized method. For the analysis of netupitant and its metabolites and palonosetron in plasma, a simultaneous, validated LC/MS/MS method was used.
Pharmacokinetic analysis
Plasma concentration–time data were analyzed by noncompartmental methods using WinNonlin Professional Edition (Pharsight Corp., Mountain View, CA, USA), to obtain values for the PK parameters as appropriate for each study.
Primary PK parameters of interest in determining drug interactions included maximum observed plasma concentration (C max), area under the plasma concentration–time curve from administration to the last sampling point (AUClast), and the AUC extrapolated to infinity (AUCinf). Additional parameters, including the time to maximum concentrations (t max) and the terminal elimination half-life (t 1/2), were determined.
Log-transformed (natural logarithms) values of the primary PK characteristics, C max, AUClast, and AUCinf were subjected to an ANOVA with fixed factors for sequence, period and treatment, and a random factor for subject within sequence. For AUCinf and C max, the parametric point estimators for the treatment ratio test/reference and the 90 % confidence intervals (CIs) were calculated using the least square means from the ANOVA of log-transformed data with subsequent exponential transformation.
Results
Pharmacokinetics
The netupitant/palonosetron DDI study
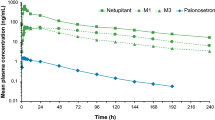
The exposure to palonosetron, in terms of C max and AUC, was similar after administration of 0.75 mg palonosetron alone and in combination with 450 mg netupitant, suggesting that netupitant did not affect the PK of palonosetron (Table 3). In general, the ratios indicate that exposure to palonosetron was slightly higher in subjects treated with palonosetron plus netupitant compared to palonosetron alone, but not significantly according to bioequivalence standards (90 % CI within the pre-defined no-effect limits of 80–125 %). Netupitant coadministration did not have any relevant effects on palonosetron metabolites. Mean metabolite to parent ratios for AUCinf were 9 and 6 % for M4 and M9, respectively, for palonosetron plus netupitant and 11 and 7 %, respectively, for palonosetron alone.
The exposure to netupitant, in terms of C max and AUC, was similar after administration of netupitant alone and in combination with palonosetron, demonstrating that palonosetron did not affect the PK of netupitant (Table 3). Similarly, the PK of the netupitant metabolites, M1, M2, and M3 was not affected by concomitant palonosetron administration. The metabolite to parent ratios for AUCinf were 32, 12, and 33 % for M1, M2, and M3, respectively, for palonosetron plus netupitant and 32, 11, and 32 %, respectively, for netupitant alone.
NEPA/ketoconazole-rifampicin DDI study
Ketoconazole effect on NEPA
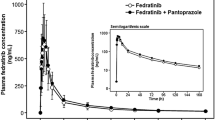
After administration of NEPA in combination with the CYP3A4 inhibitor ketoconazole, exposure to netupitant was higher compared to NEPA alone, with increases of 80 % for AUClast and 140 % for AUCinf. The effect of ketoconazole on netupitant C max was smaller with an increase of 25 % compared to NEPA alone. The 90 % confidence intervals for the PK parameters demonstrated significantly higher exposure to netupitant following NEPA coadministration with ketoconazole compared to NEPA alone and were outside the predefined no-effect limits (Table 4).
In terms of the netupitant metabolites, the formation of M1 and M3 was delayed when NEPA was coadministered with ketoconazole compared to NEPA alone (median t max of about 96 vs. 12 h, respectively, for M1 and 24 vs. 12 h, respectively for M3). Concomitant ketoconazole did not appear to have an impact on the time of the appearance of M2 in plasma. For all three metabolites (M1, M2, and M3), AUCinf was higher with mean metabolite to parent ratios of 24.9, 6.4, and 15.1 %, respectively, for NEPA coadministered with ketoconazole compared to 30.3, 12.1, and 28.1 %, respectively, for NEPA alone.
AUClast, AUCinf, and C max of palonosetron were slightly higher (10–15 %) after the administration of NEPA in combination with ketoconazole compared to NEPA alone with the 90 % CIs within the predefined no-effect limits (Table 4).
Rifampicin effect on NEPA
After the administration of NEPA in combination with the CYP3A4 inducer rifampicin, exposure to netupitant was lower as compared to NEPA alone, with decreases of 82 % for AUClast and 83 % for AUCinf. The effect of rifampicin on netupitant C max was smaller with a decrease of 62 % compared to NEPA alone. The 90 % confidence intervals for the PK parameters demonstrated significantly lower exposure of netupitant following NEPA coadministration with rifampicin compared to NEPA alone (Table 4).
The formation of netupitant metabolites was accelerated when NEPA was administered with rifampicin compared to NEPA alone (median t max of approximately 6 vs. 12 h for M1, 4 vs. 5 h for M2, and 8 vs. 10 h for M3). The AUCinf of M1 and M3 metabolites was slightly lower after the combination with rifampicin with mean metabolite to parent ratios of 34.9 and 45.5 % for NEPA with rifampicin compared to 29.3 and 26.8 %, respectively, for NEPA alone. For metabolite M2, AUCinf was greater with mean metabolite to parent ratios of 176.9 % for NEPA plus rifampicin compared to 11.0 % for NEPA alone.
Slightly lower exposure levels of palonosetron were observed after NEPA administration in combination with rifampicin compared to NEPA alone, with decreases of 19 % for AUClast and AUCinf and of 15 % for C max. These differences were not considered to be clinically relevant, and the 90 % CIs were within the predefined no-effect limits of 80–125 % for C max (Table 4).
The NEPA/oral contraceptive DDI study
Maximum concentrations for levonorgestrel were not altered following concomitant administration of the oral contraceptive with NEPA. The extent of absorption of levonorgestrel, however, was significantly higher after the administration of the oral contraceptive together with NEPA compared to the oral contraceptive alone. AUClast and AUCinf were 46 and 40 % higher, respectively, and the 90 % CIs were outside the 80–125 % bioequivalence range (Table 5).
C max, AUClast, and AUCinf for ethinylestradiol were 5, 16, and 12 % higher, respectively, after administration of the oral contraceptive together with NEPA compared to administration of the oral contraceptive alone. Changes in the PKs of ethinylestradiol were not significant, and the 90 % CIs were within the bioequivalence range of 80–125 % for C max and AUCinf (Table 5).
Safety
Across studies, the majority of the AEs were mild or moderate in intensity. Headache was frequently reported in all studies (Table 6). No subjects experienced serious clinical, laboratory, or other adverse events.
One subject was discontinued from NEPA/ketoconazole-rifampicin DDI study after an elevated gamma-glutamyl transferase value was recorded after administration of NEPA in combination with ketoconazole. The event was considered mild and resolved by the last follow-up. Increases in liver enzymes are common following treatment with ketoconazole. Thus, it is concluded that this laboratory finding was not related to NEPA treatment.
Discussion
Despite the availability of current prophylactic antiemetics, many patients still suffer from CINV, particularly with regard to nausea control in the delayed phase [19]. Two pathways are thought to regulate the acute and delayed CINV phases [6] and NEPA targets both with a unique fixed dose combination of netupitant, a new, highly selective NK1 RA [15] and palonosetron, a pharmacologically distinct 5-HT3 RA [12, 13] .
In vitro evidences indicated that the main CYP enzyme involved in the metabolism of netupitant and palonosetron are CYP3A4 and CYP2D6, respectively. No inhibition or induction potential was reported for several CYP 450 isoenzymes, including CYP2C9, for both netupitant and palonosetron. Aprepitant, an NK1 RA commonly used in clinical practice, was identified as a moderate inhibitor of the CYP3A4 and as a mild inducer of both the CYP3A4 and CYP2C9 resulting in a meaningful clinical interaction with warfarin, a coumarin-based anticoagulant frequently prescribed in cancer patients [20, 21]. Based on these results, NEPA coadministration is not expected to interfere with warfarin metabolism. As reported in Lanzarotti et al. [17] netupitant is a moderate inhibitor of the CYP3A4 system.
The present three studies were conducted to establish netupitant and NEPA potential for drug–drug interaction in clinical practice. In the first study, the interaction between netupitant and palonosetron was evaluated. The second study was aimed at analyzing the effect of ketoconazole, a strong CYP3A4 inhibitor, and rifampicin, a CYP3A4 inducer, on the PK of the individual components of NEPA. In the third study, the effect of NEPA on ethinylestradiol and levonorgestrel was evaluated to establish the potential interaction if coadministered in clinical practice in women taking oral contraceptives.
The results of the netupitant/palonosetron DDI study suggested that palonosetron did not affect the PKs of netupitant or its pharmacologically active metabolites, M1, M2, and M3. Similarly, netupitant had no clinically relevant impact on the PK of palonosetron and its metabolites, M4 and M9. The PK parameters obtained for palonosetron in this study were comparable to those reported in previously conducted single dose studies without netupitant. Similar results were obtained with aprepitant, where the concomitant administration with intravenous, IV, ondansetron, palonosetron, or oral granisetron had no clinically relevant effects [22, 23]. A statistically but not clinically relevant increase in the AUC of ondansetron was reported in Blum et al. [22] where the dose of aprepitant, 375 mg, was 3-fold higher on day 1 than commonly administered in clinical practice. Casopitant, another NK1 RA whose development has been interrupted, showed no clinically relevant interaction with both IV dolasetron and granisetron although a statistically significant increase in dolasetron C max was reported [24]. The lack of interaction between casopitant and ondansetron was shown in Johnson et al. [25], where no clinically relevant PK changes were reported.
In the second study, the impact of ketoconazole or rifampicin on each component of NEPA was evaluated. A significant interaction was evident between netupitant and ketoconazole. Netupitant AUC was increased by 140 %, and the elimination half-life was significantly prolonged with concomitant ketoconazole administration. In terms of the netupitant metabolites, the formation of M1 and M3 was delayed when NEPA was coadministered with ketoconazole, compared to NEPA administered alone. Concomitant ketoconazole did not appear to have an impact on the time of the appearance of M2 in plasma. For all three metabolites, AUCinf was higher when NEPA was administered with ketoconazole compared to NEPA alone.
Coadministration of NEPA and rifampicin resulted in netupitant decreases of 82 % for AUClast and 83 % for AUCinf. The effect of rifampicin on netupitant C max was smaller with a decrease of 62 % compared to NEPA alone. The formation of metabolites was accelerated when NEPA was administered with rifampicin compared to NEPA alone. The AUCinf for M1 and M3 was slightly lower after intake of NEPA in combination with rifampicin compared to NEPA alone. For M2, mean AUCinf was greater with NEPA plus rifampicin compared to NEPA alone. As expected, ketoconazole and rifampicin did not alter significantly the pharmacokinetics of palonosetron.
The results observed in these studies are similar to published results of other NK1 RAs, aprepitant and casopitant. The AUC of 125 mg of oral aprepitant increased by 5-fold and the half-life was increased by approximately 3-fold when coadministered with ketoconazole [21, 26]. Similarly, ketoconazole increased the C max and AUClast of a single dose of 100 mg casopitant by 2.7- and 12-fold, respectively, and increased the C max of 3-day casopitant (following 150 mg on day 1 and 50 mg on days 2 and 3), by 2.5-fold on day 1 and 2.9-fold on day 3, whereas AUClast increased by 4.3-fold on day 1 and 5.8-fold on day 3 [27].
When aprepitant was coadministered with rifampicin, the AUC of aprepitant was reduced by 11-fold, and the half-life was decreased by 3-fold [21]. Similarly, rifampicin coadministration results in reductions in the AUC and C max of casopitant by 96 and 89 %, respectively [27].
The interaction of NEPA with ethinylestradiol and levonorgestrel, CYP3A4 substrates, was evaluated in the third study. The coadministration of NEPA had no effect on the exposure to ethinylestradiol, but did result in an increase in approximately 40 % of the exposure to levonorgestrel. The observed change in levonorgestrel exposure was not considered clinically relevant with regard to the safety and efficacy of the hormonal contraception. No dosing adjustments or precautions are warranted for NEPA and oral contraceptives. This is in contrast to results observed in three different studies where aprepitant was coadministered with ethinylestradiol and norethindrone or norgestimate, where a clinically relevant decrease in the plasma concentration of the oral contraceptive was reported [21].
Most of the interactions reported in this and previous studies [17] could be considered as a NK1 RA class effect (Table 7); however, the lack of an inducing effect of netupitant on the CYP3A4 and CYPDC9 could represent a safety advantage over the other NK1 RAs.
In summary, the results of these studies demonstrate no interaction between netupitant and palonosetron supporting the development of a new unique antiemetic, NEPA, targeting the two critical pathways associated with CINV. When coadministered with a strong CYP3A4 inhibitor, ketoconazole, or an inducer, rifampicin, NEPA metabolism was significantly altered. Dose adjustments may be required when strong CYP3A4 inhibitors or inducers are coadministered in clinical practice. In contrast to aprepitant, no interaction was expected between NEPA and warfarin and evident between NEPA and oral contraceptives when coadministered. Netupitant and NEPA were well tolerated in all three studies.
References
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13(4):219–227
Feyer P, Jordan K (2011) Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol 22(1):30–38
Roila F, Herrstedt J, Aapro M, Gralla RJ et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(5):v232–v243, www.mascc.org
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC et al (2012) Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncology 29(31):4189–4198, www.asco.org
NCCN Clinical Practice Guidelines in Oncology: Antiemesis Version 1. (2012) www.nccn.org
Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ (2003) Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 39(8):1074–1080
Rubenstein EB, Slusher BS, Rojas C, Navari RM (2006) New approaches to chemotherapy-induced nausea and vomiting: from neuropharmacology to clinical investigations. Cancer J 12(5):341–347
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14(10):1570–1577
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S; 99–04 Palonosetron Study Group (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98(11):2473–2482
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124, Epub 2009 Jan 8. Erratum in: Lancet Oncol. 2010 11(3):226
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17(9):1441–1449
Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, Cantoreggi S, Sebastiani S, Pietra C, Slusher BS (2010) The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335(2):362–368, Epub 2010 Aug 19
Rojas C, Slusher BS (2012) Pharmacological mechanism of 5-HT3 and tachykinin NK-1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 684(1–3):1–7, Epub 2012 Mar 9
Stathis M, Pietra C, Rojas C, Slusher BS (2012) Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 689(1–3):25–30
Rizzi A, Campi B, Camarda V, Molinari S, Cantoreggi S, Regoli D, Pietra C, Calo' G (2010) In vitro and in vivo pharmacological characterization of the novel NK(1) receptor selective antagonist, Netupitant. Peptides 37(1):86–97
Giuliano C, Lovati E, Funk C, Potthast M, Pietra C, (2012) In vitro drug–drug interaction studies with the antiemetic drug Netupitant and its major metabolites (M1 and M2), involving main human cytochrome P450 isoenzymes. Annals of Oncology 23 (Supplement 9): ix499–ix527, doi:10.1093/annonc/mds416 (abstract 1618)
Lanzarotti C, Rossi G (2013) Effect of netupitant, a highly selective NK1 receptor antagonist, on the pharmacokinetics of midazolam, erythromycin, and dexamethasone. Support Care Cancer (this issue), doi:10.1007/s00520-013-1855-y
Palonosetron package insert (2012) Lugano, Switzerland. Helsinn Healthcare
Salsman JM, Grunberg SM, Beaumont JL, Rogers M, Paul D, Clayman ML, Cella D (2012) Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Canc Netw 10(2):149–157
Aapro MS, Walko CM (2010) Aprepitant: drug–drug interactions in perspective. Ann Oncol 21(12):2316–2323. doi:10.1093/annonc/mdq149, Epub 2010 May 20. Review
EMEND (package insert) (2013) Merck Sharp & Dohme Corp., a subsidiary of Merck & CO., Inc., Whitehouse Station
Blum RA, Majumdar A, McCrea J, Busillo J, Orlowski LH, Panebianco D, Hesney M, Petty KJ, Goldberg MR, Murphy MG, Gottesdiener KM, Hustad CM, Lates C, Kraft WK, Van Buren S, Waldman SA, Greenberg HE (2003) Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects. Clin Ther 25(5):1407–1419
Shah AK, Hunt TL, Gallagher SC, Cullen MT Jr (2005) Pharmacokinetics of palonosetron in combination with aprepitant in healthy volunteers. Curr Med Res Opin 21(4):595–601
Adams LM, Johnson B, Zhang K, Yue L, Kirby LC, Lebowitz P, Stoltz R (2009) Effect of casopitant, a novel NK-1 antagonist, on the pharmacokinetics of dolasetron and granisetron. Support Care Cancer 17(9):1187–1193
Johnson B, Adams L, Lu E, Zhang K, Lebowitz P, Lates C, Blum R (2009) Impact of casopitant, a novel NK-1 antagonist, on the pharmacokinetics of ondansetron and dexamethasone. Support Care Cancer 17(9):1177–1185
Sanchez RI, Wang RW, Newton DJ, Bakhtiar R, Lu P, Chiu SH, Evans DC, Huskey SE (2004) Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos 32(11):1287–1292
Johnson BM, Adams LM, Zhang K, Gainer SD, Kirby LC, Blum RA, Apseloff G, Morrison RA, Schutz RA, Lebowitz PF (2010) Ketoconazole and rifampin significantly affect the pharmacokinetics, but not the safety or QTc interval, of casopitant, a neurokinin-1 receptor antagonist. J Clin Pharmacol 50(8):951–959
Acknowledgments
We thank Silvia Olivari Tilola, Helen Pentikis, Norman Nagl, Jennifer Vanden Burgt, Paul Ricigliano, and Silvia Sebastiani for their support in the manuscript preparation and Claudio Giuliano for both the inclusion of the preclinical data and for his expert advises.
Disclosure
This study was sponsored by Helsinn Healthcare SA. Anders Henriksson, Klaus Peter Kammerer, and Wolfgang Timmer received funding from Helsinn Healthcare to conduct this study. All other authors were employees of Helsinn Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calcagnile, S., Lanzarotti, C., Rossi, G. et al. Effect of netupitant, a highly selective NK1 receptor antagonist, on the pharmacokinetics of palonosetron and impact of the fixed dose combination of netupitant and palonosetron when coadministered with ketoconazole, rifampicin, and oral contraceptives. Support Care Cancer 21, 2879–2887 (2013). https://doi.org/10.1007/s00520-013-1857-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1857-9