Abstract
Background
Bone metastases from various cancers have been traditionally treated with bisphosphonates, such as zoledronic acid (ZA), to prevent future skeletal-related events (SREs). Denosumab (Dmab) has been shown to have more advantages in preventing SREs in clinical trials than ZA, but the cost to administer Dmab is significantly higher.
Methods
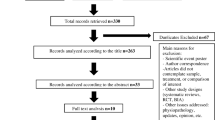
A literature review was conducted to investigate the methodologies used to compare the cost-effectiveness of Dmab and ZA. MEDLINE® and EMBASE were searched systematically for all cost-effectiveness analyses published between January week 1, 2006 to August week 1, 2012. Search strategies were designed to retrieve articles analyzing the cost-effectiveness and cost utility of Dmab compared to ZA in patients with bone metastases. From 12 references obtained in the initial database search, eight satisfied the predetermined criteria for full article review. Articles were analyzed for incremental costs per skeletal-related event avoided or incremental cost per quality-adjusted life year gained.
Results
All the studies identified received funding from Novartis Pharmaceuticals (the manufacturer of ZA) or Amgen Incorporated (the manufacturer of Dmab). The studies looked at the economic analysis using different associated costs and over various time periods, ranging from a 1-year to a lifetime time horizon.
Conclusion
It is not clear whether the methods used across studies are consistent, which may account for the differences between estimated costs and effects. Future research is suggested to explore the cost-effectiveness between Dmab and ZA using a standardize time frame and endpoint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that bone metastases develop in up to 80 % of patients with advanced prostate or breast cancer and up to 40 % of patients with other types of advanced cancers [1–3]. Extensive metastases to the bone can induce bone destruction, thereby resulting in skeletal-related events (SREs) such as hypercalcemia, pathological fractures, spinal cord compression, radiation treatment to bone, and surgery to bone [4]. SREs secondary to bone metastases can be painful and debilitating, which cause a reduction in health-related quality of life (QoL). SREs are associated with an increased cost of treatment [5] and an overall decrease in survival [6, 7]. Bisphosphonates, when used in conjunction with systemic chemotherapy, have lead to a significant reduction and delay of the incidence of SREs [8]. Zoledronic acid (ZA) has been used for preventing skeletal fractures in patients with cancer and also for the treatment of osteoporosis. The 4-week cost of treatment with ZA 4 mg was estimated to be $953 [9].
In phase III international randomized controlled trials, breast [10] and prostate [11] cancer patients with bone metastases were randomly assigned to received Denosumab (Dmab) or ZA every 4 weeks. The primary endpoint was the time to the first SRE, and subsequent on-study SREs were considered as secondary endpoints. The study found that Dmab delayed the time to the first SRE significantly in comparison to ZA (hazard ratio, 0.82) in both breast and prostate cancers, despite there being no significant difference in overall survival and disease progression in either; ZA resulted in higher rates of adverse events. The superior prevention of SREs by Dmab has led to the use of this drug for advanced breast and prostate cancer patients with bone metastases that are at risk for bone-related injuries [12]. Dmab is more expensive than ZA with a total cost of $1,672/subcutaneous injection every 4 weeks [13].
The objective of this analysis was to review literature that examines the cost-effectiveness of Dmab versus ZA with the intent of examining the differences in cost-effectiveness methodology and results. We hypothesized that the cost-effectiveness of Dmab can be influenced by the approaches and methods chosen for cost analyses. This review will be beneficial in determining whether the increased cost of Dmab is justifiable, despite additional benefits to the patient, when compared to treatment with ZA.
Methods
Ovid MEDLINE® and Ovid EMBASE were searched systematically for all cost-effectiveness analyses published between January week 1, 2006 to August week 1, 2012. In 2006, the results of the phase III clinical trials demonstrated Dmab’s superior efficacy in delaying the first and subsequent SREs compared with zoledronic acid in patients with breast cancer and bone metastases [10]. Studies published in 2006 and onwards were included to ensure that the appropriate articles were captured.
A search strategy was designed to retrieve articles analyzing the cost-effectiveness of Dmab compared to ZA in patients with bone metastases. The following keywords or medical subject headings were used: (“denosumab” OR “dmab”) AND (“zoledronic acid” OR “zoledronate”) AND (“cost-effectiveness” OR “cost-analysis”). Two independent authors (KK and KL) reviewed the titles and abstracts generated from this literature search. Full-text versions of these selected articles were identified for subsequent review according to the inclusion criteria. Inclusion criteria included published journal articles, abstracts, and review articles written in English. Exclusion criteria included duplicate articles, articles not written in English, and articles without published abstracts. In addition, to ensure that all relevant studies were captured in this review, reference lists from published manuscripts and review articles were also explored.
Each paper was reviewed and analyzed based on the perspective, time horizon, country of origin, associated costs and discounts, resources used to supplement assumptions, cost analysis endpoints, the source of funding for the study, and final conclusion regarding its effectiveness.
Results
Overview
Twelve initial articles and abstracts in the search were identified. Of the 12 articles, eight were identified to meet the inclusion criteria outlined in the “Methods” section [14–21]. Five of the studies [14, 17–20] identified received funding support from Novartis Pharmaceuticals (the manufacturer of ZA); three of the studies [15, 16, 21] were funded by Amgen Incorporated (the manufacturer of Dmab). All the studies [14, 17–21] were conducted in the USA, using data from the US perspective, except for two (Ford and Lothgren) that were conducted in the UK and Netherlands, from the UK and Netherlands perspective, respectively. None of the studies identified approached the analysis from a non-payer (societal) perspective. Two studies conducted the cost-effectiveness analysis on patients with breast cancer with bone metastases [14, 18]; five studies were conducted on patients with prostate cancer with bone metastases [15–20]. A study by Stopeck et al. [21] was an analysis for both breast and prostate cancers. The studies used different associated costs over various time periods, ranging from a 1-year to a lifetime time horizon.
All studies used a Markov decision model with variable time horizons; their calculations and transitions among health states was based on probabilities derived from the phase III clinical trial and supplemented with literature estimates. Each study followed the same treatment comparison of 120 mg Dmab given subcutaneously and 4 mg ZA given intravenously based on the previously conducted phase III trials [10, 11]. A summary of the results is given in Table 1.
Study horizons
Three different time frames were used amongst the eight studies meeting inclusion criteria. Studies conducted by Ford, Stopeck, and Lothgren used a lifetime model; Snedecor used a 27-month perspective; and Xie and Yu used a 12-month perspective. Each drug company tended to use consistent time frames between different analyses. Studies sponsored by Amgen [15, 16, 21] used a lifetime time frame when comparing Dmab to ZA. In contrast, studies that were funded by Novartis [14, 17–20] utilized a short-term time horizon (12 or 27 months).
Novartis-funded studies that conclude that Dmab is not cost-effective when compared to ZA
A study by Snedecor et al. on skeletal metastases secondary to prostate cancer [17] inferred the following from their clinical trials: Dmab in comparison to ZA was reported having fewer patients experiencing equal to greater than one SRE (−0.25; 1.04 vs. 1.29), and fewer SREs were estimated overall. Fewer SREs resulted in an increase in quality-adjusted life years (QALYs) gained (+0.00432; 0.93512 vs. 0.93080) and fewer SRE-related costs (−$1,924; $7,604 vs. $9,528). Dmab’s overall drug costs exceeded that of ZA ($5,390; $27,881 vs. $22,491). Snedecor et al. also conducted a similar analysis on skeletal metastases secondary to breast cancer [14] and inferred that Dmab, in comparison to ZA, had fewer patients experiencing equal to greater than one SRE (−0.298; 0.68 vs. 0.98) and fewer SREs were estimated overall. Fewer SREs resulted in an increase in QALYs gained (+0.0102; 0.9406 vs. 0.9305) in addition to fewer SRE-related costs (−$2,016; $5,036 vs. $7,052). Benefits were significant in preventing SREs; however, Dmab’s overall drug costs exceeded that of ZA ($7,107; $30,063 vs. $22,956). The incremental cost-effectiveness ratio (ICER) per QALY gained was $697,499 and $1,248,051 for breast and prostate cancer, respectively. Using the $100,000 ICER threshold, Dmab is not cost-effective when compared to ZA. Dmab has a higher likelihood of being a cost-effective alternative to ZA at willingness-to-pay thresholds of ≥ $600,000/QALY.
Xie et al. [20] used a nine-state Markov model using only literature-derived direct medical costs for prostate cancer. The cost per SRE avoided was evaluated over 1- and 3-year periods. Each Markov cycle was 13 weeks, with the assumption that no more than one SRE could occur within a cycle. A discount rate of 3 % was applied for both cost and effectiveness outcomes in the base case.
In the base case, the total costs incurred over 1 year were estimated at $27,528 for ZA, and the costs for Dmab were $35,341. This resulted in a 1-year incremental cost of $7,813 and a 3-year incremental cost of $13,856 for Dmab. Patients treated with Dmab were estimated to experience 0.11 (0.49 vs. 0.60) fewer SREs over the course of 1 year and 0.27 (1.18 vs. 1.46) over 3 years. Over the 1- and 3-year periods, the estimated costs per SRE avoided with the use of Dmab were $71,027 and $51,319, respectively. Based on the cost-effectiveness thresholds of $70,000, $50,000, and $30,000 per SRE avoided, Dmab was cost-effective in 49.5, 17.5, and 0.3 % of the cases at 1 year, respectively. For the 3-year scenario, Dmab was cost-effective when compared to ZA in 79.0, 49.8, and 4.1 % of the cases. The authors concluded that Dmab was not a cost-effective treatment alternative to ZA [20].
In a similar study looking at breast cancer with metastases to bone, Xie et al. [18] conducted a study where the ICER was measured as the total incremental cost per SRE avoided. Each Markov cycle was 4 weeks with the assumption that no more than one SRE could occur within a cycle. Treatment costs for the various SREs were estimated using the study by Barlev et al. Health resources normally used in real-world practice provided the costs of treatment for related adverse events.
The 1-year cumulative drug cost for ZA was $23,511 and for Dmab was $30,033, resulting in a 1-year incremental cost of $6,522. Patients treated with Dmab were estimated to experience 0.06 (0.42 vs. 0.48) fewer SREs over the course of 1 year, and the estimated incremental total direct costs per SRE avoided with the use of Dmab was $114,628. Additionally, patients treated with Dmab were estimated to experience 0.02 (0.28 vs. 0.30) fewer pathological fractures over a 1-year period. Thus, the estimated incremental total direct cost per pathological fracture avoided was $290,136 for Dmab when compared with ZA. From the sensitivity analysis carried out, the results were generally robust with the majority of variations leading to an incremental cost per SRE/pathological fracture avoided greater than $50,000. Based on the cost-effectiveness thresholds of $70,000, $50,000, and $30,000 per SRE avoided, Dmab was cost-effective in 43.6, 29.5, and 17.1 % of the cases at 1 year, respectively. Based on the cost-effectiveness thresholds of $70,000, $50,000, and $30,000 per pathological fracture avoided, Dmab was cost-effective in 27.7, 13.3, and 7.9 % of the cases at 1 year, respectively. The authors concluded that Dmab was even less cost-effective in treating breast cancer with bone metastases than prostate cancer with bone metastases in their previous study [20].
Yu et al. [19] assessed the cost-effectiveness on the basis of incremental cost per SRE avoided over the course of 1 year. The total cost for Dmab and ZA over a 1-year time horizon was estimated at $37,854 and $30,499, respectively, and Dmab had a total incremental cost of $7,355. Dmab patients were estimated to have 0.11 fewer SREs in 1 year (0.67 for ZA, 0.56 for Dmab), therefore producing an incremental cost per SRE avoided of $66,864. The sensitivity analysis demonstrated that the incremental cost per SRE avoided was most affected by the difference in the drug costs, risk of progression, and risk of first SRE (outcome values were not provided). Yu et al. concluded that despite Dmab being more effective in delaying SREs than ZA, it is not a cost-effective alternative [19].
Amgen-funded studies that conclude that Dmab is cost-effective when compared to ZA
Lothgren et al. assessed Dmab and ZA in patients with bone metastases from solid tumors using a three-state Markov model (on treatment, off treatment, and dead) for each cancer type (breast, prostate, or other) over the rest of the patient’s lifetime [16]. SRE costs and administration costs were based locally, and costs were discounted at 4 % and QALY outcomes were decreased by 1.5 % (discounts applied in the Netherlands). The EQ-5D, standardized instrument used to measure health utilities, was completed by the patients every 4 weeks to calculate baseline patient health state utility. In comparison to ZA, Dmab resulted in 0.158 (1.550 vs. 1.708) fewer SREs; 0.013 more QALYs (1.380 vs. 1.368); and lower SRE-related costs but higher total cost (€542; €11,912 vs. €11,370) for breast cancer, prostate cancer, and other solid tumors, respectively. Administration cost, SRE and adverse event cost, and SRE QALY decrements were varied in a one-way sensitivity analysis. Dmab is more effective than ZA in preventing SREs over the rest of the patients’ lives. In non-UK European countries, the traditional threshold per QALY gained is €50,000, thus making an incremental cost of €26,524, €44,622, or €11,660 a cost-effective alternative.
Stopeck et al. [21] chose to analyze the cost-effectiveness of Dmab versus ZA using a 28-day-cycle lifetime Markov model based on the clinical trials reported by Stopeck et al. [10] and Fizazi et al. [11], involving patients with castration-resistant prostate cancer, breast cancer, and non-small-cell lung cancer and bone metastases. This study looked at the ICER per QALY gained as well as ICER per SRE avoided. A large commercial database provided real-world SRE rates in ZA-treated patients. SRE and treatment administration QALY decrements were estimated with time trade-off studies. Drug, drug administration, and renal monitoring costs were included. All costs and QALYs were discounted at 3 % annually. Stopeck et al. incorporated real-world retrospective claim data and used an adjustment factor of 2.01 for the SRE rates of ZA-treated patients in the model. The rates for Dmab-treated patients were established by applying the treatment effects of the phase 3 clinical trials [10, 11]. Stopeck et al. also looked at separate scenarios where they considered costs, taking into consideration drug discontinuation and adverse events. QALY-associated decrements were derived from a time trade-off study in UK assessing the utility of different health states of a hypothetical patient cohort with bone metastases.
In the base case using their adjusted rates, Dmab-treated patients with prostate cancer experienced on average 0.81 (3.23 vs. 4.04) fewer SREs, gained 0.14 more QALYs (0.97 vs. 0.83), and acquired $6,910 greater total lifetime costs ($76,486 vs. $69,577). This resulted in an incremental cost per QALY gained and SRE avoided of $49,405 and $8,567, respectively [21]. Dmab was concluded to be cost-effective based on the cost per QALY thresholds of $100,000, $150,000, and $200,000, the probabilities of Dmab being cost-effective were 83, 94, and 98 %, respectively [21].
Dmab-treated patients with breast cancer experienced an average of 0.99 (3.56 vs. 4.55) fewer SREs, gained 0.17 more QALYs (1.76 vs. 1.59), and acquired $13,451 greater total lifetime costs ($108,538 vs. $95,087). This resulted in an incremental cost per QALY gained and SRE avoided of $78,915 and $13,557, respectively. Dmab was concluded to be cost-effective based on the cost per QALY thresholds of $100,000, $150,000, and $200,000; the probabilities of Dmab being cost-effective were 62, 79, and 91 %, respectively [21].
Commissioned by the National Institute for Health and Clinical Excellence (NICE), Ford et al. presented a partially adapted cost-effectiveness analysis comparing Dmab to ZA. Ford et al. [15] applied their modified assumptions to Amgen’s previously conducted analysis. A lifetime Markov model was used which included: SRE-related costs, quality of life effects of SRE from the clinical trial with EQ-5D, and drug and administration costs were estimated based on an unpublished micro costing study in the UK. For this analysis, only “SRE-experienced” patients were assessed in this situation. The study estimated that patients treated with Dmab compared with zoledronic acid experienced fewer SREs (0.14; 1.98 vs. 2.12) and more QALYs (0.006; 1.089 vs. 1.083). When including NICE-approved patient access scheme (PAS) which allows patient access to high-cost therapies thereby improving the cost-effectiveness, Dmab was demonstrated to be more cost saving and to dominate ZA. Ford et al. extrapolated the study data to include both the SRE-experienced and SRE-naïve patients. When including PAS, Dmab was cost-effective in both populations [15].
Discussion
Phase III clinical trials have confirmed the effectiveness of both Dmab and ZA in patients with bone metastatic breast and prostate cancer [10, 11]. This is a summary of the current cost-effectiveness analyses between Dmab and ZA with the intention of identifying inconsistencies that may account for the discrepancy in study conclusions regarding the overall cost-effectiveness of the drugs.
Based on the eight studies reviewed and their respective methodologies, five studies [14, 17–20] indicated that although benefits were gained through preventing and delaying SREs by treatment with Dmab, the high costs of this drug do not outweigh the similar benefits observed when using ZA. Contrarily, the cost-effectiveness analysis results from three studies (Ford et al., Lothgren et al., and Stopeck et al.) indicated that Dmab has superior efficacy, favorable safety, and effective administration, making it a more cost-effective choice when compared to ZA [15, 16, 21].
We compared current literature on the cost-effectiveness of Dmab when compared to ZA in the UK, Netherlands, and USA. The studies conducted from a Dutch [16] and UK [15] perspective concluded that Dmab was more cost-effective when compared to ZA. Only one study, conducted by Stopeck et al. [21], from the USA had similar conclusion. The differences in health-care system structure between each of these countries challenge the extent to which we can make effective comparisons between these drugs. In addition, some countries may offer their patients discounts on high-cost drugs, such as a PAS in the study by Ford et al. [15], which would have an impact on the cost-effectiveness analysis. The location of the study affects the drug acquisition costs, the available discounts, and thresholds for justifiable treatment, which all play an integral role in the methodology that estimates the projected costs for treatment.
When categorizing the studies according to drug company affiliations, each drug company tended to use consistent time frames between different analyses. Studies sponsored by Amgen [15, 16, 21] used a lifetime time frame when comparing Dmab to ZA. In contrast, studies that were funded by Novartis [14, 17–20] utilized a 12- or 27-month perspective. This discrepancy plays a role in the determination of ICER since the models that use a 1-year time horizon do not take into consideration the associated costs and reductions of QoL due to SREs that occur after the period of interest, thereby overestimating the ICER for Dmab. If the time frame is too short, the determination of a drug to be either cost-effective or cost-ineffective may not be realized. Hence, it is important to differentiate between the incremental costs per SRE avoided metric and the incremental cost per QALY gained, which incorporates the timing of the SRE and the duration of their effect [22].
The barriers to access and affordability of Dmab may increase in the year 2013, as the generic pricing of ZA will take its effect. As a result, the overall cost-effectiveness will need to be reassessed with the new pricing which may skew the results presented within the current literature. Since Dmab was approved in 2010, its patent will extend for a period of about 12 years. After this point, Dmab will become a generic drug, and future cost-effectiveness analyses should compare differences to assess changes in results.
Previous studies [22, 23] have postulated that possible over-identification of asymptomatic vertebral fractures would overestimate the value of SRE-limiting agents that largely reduce asymptomatic events in patients with advanced disease. This overestimation of the real-world incidence of SREs could impact the estimated cost-effectiveness values of SRE-limiting agents and may be optimistically low. Similarly, clinical trials of SRE-limiting agents tend to underreport the incidence rate of SREs. This would be as a result of the accrual of patients that tend to be healthier with little disease progression. Hence, the sample of patients for these clinical trials does not accurately represent this patient population, as incidence rates of SRE may actually be higher than reported. Hatoum et al. and Rader et al. have suggested that future-modeled cohorts should be composed of patients with advanced disease and an increased risk of experiencing an SRE to accurately emulate a real-world approach [24, 25].
The use of the Markov model is limited since it is based on clinical trials in a controlled setting [22]. To improve the external validity of the study, these analysis models should also be based on real-world data. Moreover, recent sensitivity analyses have indicated that health economic endpoints are most receptive to drug acquisition costs and assumptions regarding patient survival and QoL [22]. Therefore, minor differences in assumptions made can have a significant impact on the cost-effective comparisons. All of the reviewed analyses were conducted and reported from a narrow group of researchers; therefore, similar models and funding sources were utilized almost exclusively from the pharmaceutical companies that manufacture Dmab and ZA [22].
Amongst all of the cost-effectiveness analyses, many experts largely criticized both studies of Xie et al. [18, 20] due to the extensive use of assumptions in these models. This may result in a high degree of uncertainty in the conclusion drawn from this work. In both studies, it was assumed that only one SRE could occur within a given cycle. Although the literature suggests that the mean time between the first and second type of SRE was approximately 3 months, it is possible that two separate SREs could occur in one cycle, creating a complication for the cost analysis and the impact on the patient’s QoL. QoL was another issue that was not considered in Xie’s assessment, as criticized by Rader et al. [24]. Xie’s studies assess cost-effectiveness from the perspective of cost per SRE avoided but do not look into the QALY gained, which is highly dependent on the anticipated number and severity of SREs. The model that Xie utilized pulls data from a variety of different sources, which could lead to potential bias in the study results.
It was also suggested by Carter et al. [22] that future investigations should determine the cost-effectiveness of SRE-limiting agents in SRE-experienced and SRE-naïve subgroups independently since Ford’s study showed that these patient populations have differences in survival and QoL measures. Another solution would be to adjust the models to reflect the real-world portion of SRE-naïve and -experienced patients, so that there is a fair representation of the entire patient cohort. Future studies should utilize the cost per QALY metric since it is a more sensitive and meaningful measure and is also considered one of the primary endpoints of care for patients with advanced cancers. Studies should consider the imminent changes in generic pricing for ZA and the subgroups that may derive the greatest economic benefit from SRE-limiting therapy.
Conclusion
The current review has investigated eight studies analyzing the cost-effectiveness of denosumab against zoledronic acid as a treatment to avoid and delay SREs in patients with advanced cancers metastatic to the bone. Due to variability in study endpoints, associated costs and discounts, and time horizons, opposing conclusions were reached by the studies, which were funded by the different drug manufacturers. Consensus among the researchers may be needed to outline the agreed optimal approach in the cost-effectiveness analysis. By finding a standardized approach to summarizing the available evidence, clinicians and policy makers will be better informed of the current literature when deciding on the best treatment options for their patients.
References
Coleman RE, Guise TA, Lipton A et al (2008) Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res 14(20):6387–6395
Coleman RE (2008) Risks and benefits of bisphosphonates. Br J Cancer 98(11):1736–1740
Janjan N. Bone metastases: approaches to management. Sem Oncol 2001; 28 (4 Suppl. 11): 28–34
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9(Suppl 4):14–27
Pockett R, Castellano D, McEwan P et al (2010) The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 19:755–760
Sathiakumar N, Delzell E, Morrisey MA et al (2011) Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 14(2):177–183
Bouganim N, Clemons MJ (2011) Bone-targeted agents in the treatment of bone metastases: RANK outsider or new kid on the block? Future Oncol 7(3):381–383
Costa L, Lipton A, Coleman RE (2006) Role of bisphosphonates for the management of skeletal complications and bone pain from skeletal metastases. Support Cancer Ther 3(3):143–153
Readyprice S (2010) Red book 2010: pharmacy’s fundamental reference (Red book drug topics). Thomson Healthcare, Montvale
Stopeck A, Lipton A, Body J et al (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer, a randomized, double blind study. J Clin Oncol 28(35):3152–3159
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377(9768):813–822
Dranitsaris G, Hatzimichael E (2012) Interpreting results from oncology clinical trials: a comparison of denosumab to zoledronic acid for the prevention of skeletal-related events in cancer patients. Support Care Cancer 20:1353–1360
Zgeva (denosumab) for subcutaneous injection. Amgen. Revised 2010. Available at: http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=79486
Snedecor SJ, Carter JA, Kaura S, Botteman MF (2012) Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther 34(6):1334–1349
Ford J, Cummins E, Sharma P et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumors. Aberdeen: Aberdeen Health Technology Assessment Group, Institute of Applied Sciences, University of Aberdeen; 2011
Lothgren M, Bracco A, Lucius B et al (2011) Cost-effectiveness of denosumab vs. zoledronic acid (ZA) for the prevention of skeletal related events (SRE) in patients with bone metastases from solid tumors in the Netherlands. Value Health 14:A455
Snedecor SJ, Carter JA, Kaura S et al. Cost-effectiveness of zoledronic acid (ZOL) versus denosumab (Dmab) in prevention of skeletal-related events (SREs) in castration-resistant prostate cancer metastatic to the bone (mCRPC). J Clin. Oncol. 2011;(Suppl.) S29.
Xie J, Diener M, Sorg R et al (2012) Cost-effectiveness of denosumab compared with zoledronic cid in patients with breast cancer and bone metastases. Clin Breast Cancer 12(4):247–258
Yu A, Namjoshi J, Xie K et al. Economic evaluation of denosumab compared with zoledronic acid in patients with hormone-refractory prostate cancer with bone metastases [Abstract]. Paper presented at the American Society of Clinical Oncology Genitourinary Cancer Symposium. Chicago, IL, USA, 3–7 June 2011.
Xie J, Namjoshi M, Wu EQ et al (2011) Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 17(8):621–643
Stopeck A, Rader M, Henry D et al (2012) Cost-effectiveness of denosumab vs. zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 15(4):712–723
Carter JA, Botteman MF (2012) Health-economic review of zoledronic acid for the management of skeletal related events in bone-metastatic prostate cancer. Expert Rev Pharmacoecon Outcome Res 12(4):1–13
Sartor O (2011) Denosumab in bone-metastatic prostate cancer: known effects on skeletal-related events but unknown effects on quality of life. Asian J. Androl 13(4):612–613
Rader M, Goessl C, Cong Z (2012) Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 18(1):74–75
Hatoum HT, Lin SJ, Smith MR, Barghout V, Lipton A (2008) Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 113(6):1438–1445
Acknowledgments
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Conflicts of interest
Dr. Nicole Mittmann has acted as a consultant to both Amgen Canada and Novartis Canada. Dr. Edward Chow has research and educational projects with both Amgen and Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, K., Lam, K., Mittmann, N. et al. Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer 21, 1785–1791 (2013). https://doi.org/10.1007/s00520-013-1790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1790-y