Abstract
Purpose
Acute respiratory failure that requires invasive mechanical ventilation is a leading cause of death in critically ill cancer patients. The aim of this study was to evaluate the outcome and prognostic factors of patients requiring invasive mechanical ventilator for acute respiratory failure, within 1 month of ambulatory chemotherapy for solid cancer.
Methods
A retrospective observational study of patients who underwent ambulatory chemotherapy at Samsung Medical Center, between January of 2007 and April of 2009, was employed for this study.
Results
A total of 51 patients met the inclusion criteria and were included in the study. The median age was 65 years (25–87) and the majority of the patients were male (n = 38, 74.5 %). There were 42 patients (82.3 %) with lung cancer. The most common cause of acute respiratory failure was pneumonia (n = 24, 47.1 %), followed by acute respiratory failure due to extra-pulmonary infection, drug-induced pneumonitis, alveolar hemorrhage, and cancer progression. The intensive care unit (ICU) mortality was 68.6 % and the most common cause of death in the ICU was uncorrected cause of acute respiratory failure. Before adjustment for others factors, prechemotherapy Eastern Cooperative Oncology Group (ECOG) Performance Scale (PS) (P = 0.03), Sequential Organ Failure Assessment score (P = 0.01), and anemia (P = 0.04) were significantly associated with ICU mortality. However, when adjusted for age, sex, and Acute Physiologic and Chronic Health Evaluation II score, only poor ECOG PS (≥2) was significantly associated with ICU mortality [OR 6.36 (95 % CI (1.02–39.5))].
Conclusions
The outcome of patients with acute respiratory failure needing invasive mechanical ventilation during ambulatory chemotherapy for solid cancer is poor. Prechemotherapy performance status is an independent predictor of mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival rate of critically ill cancer patients has improved over the past two decades [1–3]. The reasons for this improvement likely include advances in the management of organ failure in the intensive care unit (ICU) [4–6], the use of non-invasive mechanical ventilation [5, 7] and the introduction of new treatments for solid tumors or hematologic malignancy [8, 9]. However, acute respiratory failure with the need for invasive mechanical ventilation still remains the leading cause of death in critically ill cancer patients [10–12]. Consequently, when a cancer patient faces acute respiratory failure, mechanical ventilation is often considered futile [13–15].
Cytotoxic chemotherapy is being administered in an increasing number of solid cancer patients [16] on an intermittent drug dose schedule, allowing ambulatory treatment. While undergoing ambulatory chemotherapy, patients may develop respiratory failure, requiring mechanical ventilation. Approximately 5 % of patients with solid cancers experience acute respiratory failure during the course of their disease [17, 18]. However, to date, the outcome and prognostic factors specific for this patient population have not been reported in the literature. Here, we evaluated the outcome and prognostic factors of acute respiratory failure requiring invasive mechanical ventilation in solid cancer patients within one month of ambulatory chemotherapy.
Methods
Patients
This retrospective study included patients admitted to the medical ICU of Samsung Medical Center (a university-affiliated, tertiary referral hospital in Seoul, Korea) between January of 2007 and April of 2009. Solid cancer patients with acute respiratory failure requiring invasive mechanical ventilation and who had received ambulatory chemotherapy within 1 month of ICU admission were included. Patients were excluded from this study when they were less than 18 years old or stayed in the ICU for less than 24 h. Permission was obtained from the Institutional Review Board of the Samsung Medical Center to review and publish information from patients’ records. Informed consent was waived because of the retrospective nature of the study.
Data collection
Epidemiological and clinical data available at the time of ICU admission were collected from patients’ medical records. Data included age, sex, smoking history, comorbid conditions, type of solid cancer, time from last dose of chemotherapy to ICU admission, performance status (PS) within the preceding week before chemotherapy, severity-of-illness scores, reason for acute respiratory failure, laboratory values, therapeutic interventions during the stay in the ICU such as vasopressor use, renal replacement therapy or tracheostomy, ICU mortality, hospital mortality, and cause of death.
PS was measured with the Eastern Cooperative Oncology Group (ECOG) Scale (PS 0: fully active; PS 1: restricted in physically strenuous activity; PS 2: ambulatory and capable of all self-care, but unable to carry out any work activities; PS 3: confined to bed or chair more than 50 % of waking hours; PS 4: bedridden) [19].
For assessment of severity-of-illness, Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores were calculated. APACHE II which was originally developed to predict hospital mortality of patients admitted to the ICU but is also used to assess severity-of-illness [20, 21]. Age, type of the ICU admission, chronic health status, and worst value of 12 physiologic variables in the first 24 h of the ICU admission are used to calculate a composite score [20, 21]. The SOFA score is a scoring system to determine the extent of a patient’s organ dysfunction [22]. The score is based on six different scores, one each for the respiratory, cardiovascular, hepatic, coagulation, renal, and neurological systems [22].
Definitions
Acute respiratory failure was defined as symptoms of respiratory distress with the need for invasive ventilator support [17]. Clinically diagnosed pneumonia was defined as the presence of a new infiltrate on chest radiography plus at least one of the following: fever (body temperature >38.2 °C) or hypothermia (temperature <35.0 °C), new cough with or without sputum production, pleuritic chest pain, dyspnea, or altered sound of breathing on auscultation, as previously described [23]. Microbiologically documented pneumonia was defined as the presence of >104 colony forming units/ml in the bronchoalveolar lavage (BAL) fluid or the presence of >105 colony forming units/ml in the endotracheal aspirate culture [24]. The presence of Pneumocystis jiroveci or Aspergillus spp. in the BAL was considered diagnostic. Viral pneumonia was diagnosed when a virus was recovered from either the BAL or the nasopharyngeal specimens in a patient with clinical features consistent with viral pneumonia [17]. Ventilator-associated pneumonia was referred to as pneumonia that arises more than 48 h after endotracheal intubation [25]. Cardiogenic pulmonary edema was diagnosed when that the echocardiography showed left ventricular dysfunction and serum B-type natriuretic peptide was elevated. Bilateral pleural effusions and rapid improvement in pulmonary status after diuresis were typically observed in patients with cardiogenic pulmonary edema [26]. Alveolar hemorrhage and pulmonary infiltration by malignancy were diagnosed using previously reported criteria [27, 28]. In patients with alveolar hemorrhage, the retrieved BAL was hemorrhagic and contained hemosiderin-laden macrophages. The diagnosis of antineoplastic agent-induced pulmonary toxicity depended upon an appropriate history of drug exposure and the exclusion of other causes of respiratory failure, including infection, cardiogenic pulmonary edema, diffuse alveolar hemorrhage and lymphangitic spread of the cancer [29, 30]. Bronchoscopy with BAL was used to exclude infectious process and diffuse alveolar hemorrhage. Leukopenia was defined as a leukocyte count of less than 1,000 cells/mm3 [31] and anemia was defined as a hemoglobin level less than 12 g/dl [32].
Statistical analyses
Data are presented as the median (interquartile range (IQR)) for continuous variables and as the number (%) for the categorical variables. Data were compared using the Mann–Whitney U test for continuous variables and the Chi square test or Fisher’s exact test for categorical variables. A logistic regression model was used to adjust for potential confounding factors in the association between prognostic factors and ICU mortality. Each variable was adjusted for age, sex and APACHE II score. Data are presented as the odds ratio (OR) with a 95 % confidence interval (CI). All tests were two-sided, and a P value of less than 0.05 was considered statistically significant. Data were analyzed using the PASW Statistics software version 15 (SPSS Inc, Chicago, IL).
Results
Patient characteristics
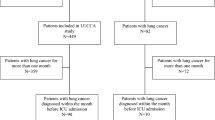
During the study period, 51 patients who met the inclusion criteria were included in the analysis. Their main demographical and clinical characteristics are summarized in Table 1. The median age was 65 (25–87) years. A majority of the patients were male (n = 38, 74.5 %). The most common primary site of solid cancer was the lung (n = 42, 82.3 %). Forty-seven patients (92.2 %) had distant metastasis. The median duration from last chemotherapy to ICU admission was 7 (3–22) days. Ten patients (19.6 %) had ECOG PS 0 or 1 and 41 patients (80.4 %) had ECOG PS 2 or 3. The median SOFA score was 8 (5–14) and the median APACHE II score was 17 (7–32). Twenty-five patients (49.0 %) received vasopressor treatment and 12 patients (23.5 %) received renal replacement therapy. The median duration of mechanical ventilation was 4.5 (1–80) days. The median duration of ICU stay was 7 (1–80) days.
Causes of acute respiratory failure
The most common cause of acute respiratory failure needing mechanical ventilation was pneumonia (n = 24, 47.1 %). Seventeen patients (33.4 %) had microbiologically documented pneumonia. Acinetobacter baumannii (n = 4, 23.5 %) and Staphylococcus aureus (n = 4, 23.5 %) were the most common pathogens. The other organisms documented were Streptococcus pneumoniae (n = 3, 17.6 %), Pseudomonas aeruginosa (n = 2, 11.8 %), Klebsiella pneumoniae (n = 2, 11.8 %), Stenotrophomonas maltophilia (n = 1, 5.9 %) and Cytomegalovirus (n = 1, 5.9 %). Eleven patients (21.5 %) had acute respiratory failure due to extra-pulmonary infection. Seven patients (13.7 %) had drug-induced pneumonitis and two patients (3.9 %) had alveolar hemorrhage, and hemoptysis and cancer progression, respectively. Other causes of respiratory failure were pulmonary embolism (n = 1, 2 %), acute exacerbation of chronic obstructive pulmonary disease (n = 1, 2 %) and acute exacerbation of idiopathic pulmonary fibrosis (n = 1, 2 %) (Table 2).
Factors associated with adverse outcome
The ICU mortality was 68.6 % (35 out of 51) and the hospital mortality was 80.4 % (41 out of 51). Most patients died due to progression of the cause of acute respiratory failure (n = 28, 80 %), while five patients (14.3 %) died due to newly acquired ventilator-associated pneumonia and two patients (5.7 %) died from arrhythmia (Table 3). ICU survivors and non-survivors were compared in Tables 4 and 5. In the unadjusted logistic regression model, ECOG PS (P = 0.03), SOFA score (P = 0.01) and anemia (P = 0.04) were significantly associated with ICU mortality (Tables 4 and 5). However, after adjusting for age, sex, and APACHE II score, only prechemotherapy ECOG PS ≥ 2 [OR 6.36 (95 % CI (1.02–39.5))] remained a significant predictor of ICU mortality (Tables 4 and 5).
Discussion
To our knowledge, this is the first study conducted specifically for this population of acute respiratory failure patients requiring mechanical ventilation while undergoing ambulatory chemotherapy for solid cancer. The ICU mortality in this population was 68.6 %, while the in-hospital mortality was 80.4 %. A poor prechemotherapy ECOG PS was the most important prognostic factor of ICU mortality in this patient group.
The ICU mortality observed in this study is consistent with previous studies of large cohorts of cancer patients receiving ICU care which report mortality ranging from 50 to 83 % [1, 5, 13, 14, 33, 34]. The mortality of cancer patients needing ICU care may vary according to patient populations, level of ICU support, severity of acute illness, and ICU policies. The relatively high ICU mortality in this study may be explained by several reasons. First, only acute respiratory failure patients needing invasive mechanical ventilator support were included. ICU cancer patients treated by invasive mechanical ventilation consistently show an extremely low survival rate [34–38]. Second, it is likely that the reasons for initiating mechanical ventilation may also have influenced the outcome. Patients with sepsis or shock-related and acute hypoxia-related respiratory failure have a worse prognosis compared to those with postoperative acute respiratory failure or cardiogenic pulmonary edema [1, 13, 17]. In this study, the two main reasons for mechanical ventilation were respiratory failure due to pneumonia and sepsis, and there were no patients with postoperative acute respiratory failure or cardiogenic pulmonary edema.
The most important finding in this study was that the ECOG PS within the preceding week before chemotherapy presented a simple, but useful, predictor of outcome in solid cancer patients with acute respiratory failure. ECOG PS (≥2) before last chemotherapy was the only statistically significant predictor of mortality after adjusting for age, sex, and APACHE II. This is consistent with other studies that reported prognostic factors in cancer patients admitted to the ICU. Christodoulou et al. [39] reported that an ECOG PS score of 3 or 4 prior to hospitalization was found to be a simple negative predictor of the short term outcome of cancer patients with solid tumors admitted to the ICU. Soares et al. [33] also demonstrated the impact of ECOG PS score on hospital mortality in general cancer patients admitted to the ICU.
Based on the results of this study, 19.6 % had ECOG PS 0 or 1 and 80.4 % had ECOG PS 2 or 3. Even though the mortality of the patients with ECOG PS ≥2 was higher than that of the patients with ECOG PS ≤1 (78 % vs 30 %), we still cannot conclude that ECOG PS ≤1 is prerequisite for the ICU admission of critically ill solid cancer patients undergoing ambulatory chemotherapy for acute respiratory failure. However, the prechemotherapy ECOG PS score may be useful for clinicians, as an aid in discussing treatment options with patients and their families.
In our patients, the cause of respiratory failure was pneumonia in about half of the patients. The bacteriology in this study was very similar to that of a healthcare-associated pneumonia, and included organisms such as A. baumannii, S. aureus, P. aeruginosa, and K. pneumoniae. In fact, 76 % of identified organisms were either methicillin-resistant S. aureus or enteric gram-negative bacilli. In these patients, empiric antibiotic therapy should include coverage for multi-resistant gram-negative bacilli and possibly methicillin-resistant S. aureus if respiratory secretions are positive for gram-positive cocci.
This study has substantial limitations. First, given its retrospective observational design, we did not have any data regarding patients who were not referred to the ICU, possibly introducing significant selection bias. Second, this study was conducted at a single center with a relatively small number of patients which limits generalization of our findings.
In conclusion, we found that the outcome of patients with acute respiratory failure needing mechanical ventilation during ambulatory chemotherapy for solid cancer was poor and that prechemotherapy ECOG PS was an independent predictor of mortality. Prechemotherapy ECOG PS should be incorporated into the discussion of treatment options with patients and their families in this patient group.
References
Kress JP, Christenson J, Pohlman AS, Linkin DR, Hall JB (1999) Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med 160:1957–1961
Azoulay E, Recher C, Alberti C, Soufir L, Leleu G, Le Gall JR, Fermand JP, Schlemmer B (1999) Changing use of intensive care for hematological patients: the example of multiple myeloma. Intensive Care Med 25:1395–1401
Staudinger T, Stoiser B, Mullner M, Locker GJ, Laczika K, Knapp S, Burgmann H, Wilfing A, Kofler J, Thalhammer F, Frass M (2000) Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med 28:1322–1328
Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, Reiffers J, Cardinaud JP (2001) Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 344:481–487
Azoulay E, Alberti C, Bornstain C, Leleu G, Moreau D, Recher C, Chevret S, Le Gall JR, Brochard L, Schlemmer B (2001) Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med 29:519–525
Larche J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, Darmon M, Le Gall JR, Schlemmer B (2003) Improved survival of critically ill cancer patients with septic shock. Intensive Care Med 29:1688–1695
Patrick W, Webster K, Ludwig L, Roberts D, Wiebe P, Younes M (1996) Noninvasive positive-pressure ventilation in acute respiratory distress without prior chronic respiratory failure. Am J Respir Crit Care Med 153:1005–1011
Bergh J, Wiklund T, Erikstein B, Lidbrink E, Lindman H, Malmstrom P, Kellokumpu-Lehtinen P, Bengtsson NO, Soderlund G, Anker G, Wist E, Ottosson S, Salminen E, Ljungman P, Holte H, Nilsson J, Blomqvist C, Wilking N (2000) Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: a randomised trial. Scandinavian Breast Group 9401 study. Lancet 356:1384–1391
Thatcher N, Girling DJ, Hopwood P, Sambrook RJ, Qian W, Stephens RJ (2000) Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol 18:395–404
Snow RM, Miller WC, Rice DL, Ali MK (1979) Respiratory failure in cancer patients. JAMA 241:2039–2042
Roques S, Parrot A, Lavole A, Ancel PY, Gounant V, Djibre M, Fartoukh M (2009) Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 35:2044–2050
Soares M, Depuydt PO, Salluh JI (2010) Mechanical ventilation in cancer patients: clinical characteristics and outcomes. Crit Care Clin 26:41–58
Groeger JS, White P Jr, Nierman DM, Glassman J, Shi W, Horak D, Price K (1999) Outcome for cancer patients requiring mechanical ventilation. J Clin Oncol 17:991–997
Epner DE, White P, Krasnoff M, Khanduja S, Kimball KT, Knaus WA (1996) Outcome of mechanical ventilation for adults with hematologic malignancy. J Investig Med 44:254–260
Kongsgaard UE, Meidell NK (1999) Mechanical ventilation in critically ill cancer patients: outcome and utilisation of resources. Support Care Cancer 7:95–99
Lokich J, Bothe A Jr, Fine N, Perri J (1982) The delivery of cancer chemotherapy by constant venous infusion. Ambulatory management of venous access and portable pump. Cancer 50:2731–2735
Azoulay E, Thiery G, Chevret S, Moreau D, Darmon M, Bergeron A, Yang K, Meignin V, Ciroldi M, Le Gall JR, Tazi A, Schlemmer B (2004) The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore) 83:360–370
Azoulay E, Schlemmer B (2006) Diagnostic strategy in cancer patients with acute respiratory failure. Intensive Care Med 32:808–822
Conill C, Verger E, Salamero M (1990) Performance status assessment in cancer patients. Cancer 65:1864–1866
Abbott RR, Setter M, Chan S, Choi K (1991) APACHE II: prediction of outcome of 451 ICU oncology admissions in a community hospital. Ann Oncol 2:571–574
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, Fernandez-Aguera A, Verdaguer R, Martinez J, Manresa F, Gudiol F (2005) Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med 142:165–172
Azoulay E, Darmon M, Delclaux C, Fieux F, Bornstain C, Moreau D, Attalah H, Le Gall JR, Schlemmer B (2002) Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med 30:781–786
Anonymous (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Higenbottam T, Kuwano K, Nemery B, Fujita Y (2004) Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer 91(Suppl 2):S31–37
De Lassence A, Fleury-Feith J, Escudier E, Beaune J, Bernaudin JF, Cordonnier C (1995) Alveolar hemorrhage. Diagnostic criteria and results in 194 immunocompromised hosts. Am J Respir Crit Care Med 151:157–163
Azoulay E, Fieux F, Moreau D, Thiery G, Rousselot P, Parrot A, Le Gall JR, Dombret H, Schlemmer B (2003) Acute monocytic leukemia presenting as acute respiratory failure. Am J Respir Crit Care Med 167:1329–1333
Vahid B, Marik PE (2008) Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 133:528–538
Limper AH (2004) Chemotherapy-induced lung disease. Clin Chest Med 25:53–64
Rhee CK, Kang JY, Kim YH, Kim JW, Yoon HK, Kim SC, Kwon SS, Kim YK, Kim KH, Moon HS, Park SH, Kim HJ, Lee S, Song JS (2009) Risk factors for acute respiratory distress syndrome during neutropenia recovery in patients with hematologic malignancies. Crit Care 13:R173
Cardenas-Turanzas M, Cesta MA, Wakefield C, Wallace SK, Puana R, Price KJ, Nates JL (2010) Factors associated with anemia in patients with cancer admitted to an intensive care unit. J Crit Care 25:112–119
Soares M, Salluh JI, Spector N, Rocco JR (2005) Characteristics and outcomes of cancer patients requiring mechanical ventilatory support for >24 hrs. Crit Care Med 33:520–526
Maschmeyer G, Bertschat FL, Moesta KT, Hausler E, Held TK, Nolte M, Osterziel KJ, Papstein V, Peters M, Reich G, Schmutzler M, Sezer O, Stula M, Wauer H, Wortz T, Wischnewsky M, Hohenberger P (2003) Outcome analysis of 189 consecutive cancer patients referred to the intensive care unit as emergencies during a 2-year period. Eur J Cancer 39:783–792
Azoulay E, Moreau D, Alberti C, Leleu G, Adrie C, Barboteu M, Cottu P, Levy V, Le Gall JR, Schlemmer B (2000) Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med 26:1817–1823
Groeger JS, Lemeshow S, Price K, Nierman DM, White P Jr, Klar J, Granovsky S, Horak D, Kish SK (1998) Multicenter outcome study of cancer patients admitted to the intensive care unit: a probability of mortality model. J Clin Oncol 16:761–770
Kroschinsky F, Weise M, Illmer T, Haenel M, Bornhaeuser M, Hoeffken G, Ehninger G, Schuler U (2002) Outcome and prognostic features of intensive care unit treatment in patients with hematological malignancies. Intensive Care Med 28:1294–1300
Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, Dal Pizzol F, Mello PV, Bozza FA, Silva UV, Torelly AP, Knibel MF, Rezende E, Netto JJ, Piras C, Castro A, Ferreira BS, Rea-Neto A, Olmedo PB, Salluh JI (2010) Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med 38:9–15
Christodoulou C, Rizos M, Galani E, Rellos K, Skarlos DV, Michalopoulos A (2007) Performance status (PS): a simple predictor of short-term outcome of cancer patients with solid tumors admitted to the intensive care unit (ICU). Anticancer Res 27:2945–2948
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.Y., Lim, S.Y., Um, SW. et al. Outcome and predictors of mortality in patients requiring invasive mechanical ventilation due to acute respiratory failure while undergoing ambulatory chemotherapy for solid cancers. Support Care Cancer 21, 1647–1653 (2013). https://doi.org/10.1007/s00520-012-1709-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1709-z