Abstract
Background
There is no consensus on whether therapeutic intensity can be reduced safely in children with low-risk febrile neutropenia (FN). Our primary objective was to determine whether there is a difference in efficacy between outpatient and inpatient management of children with low-risk FN. Our secondary objective was to compare oral and parenteral antibiotic therapy in this population.
Methods
We performed electronic searches of Ovid Medline, EMBASE, and the Cochrane Central Register of Controlled Trials, and limited studies to prospective pediatric trials in low-risk FN. Percentages were used as the effect measure.
Results
From 7,281 reviewed articles, 16 were included in the meta-analysis. Treatment failure, including antibiotic modification, was less likely to occur in the outpatient setting compared with the inpatient setting (15 % versus 28 %, P = 0.04) but was not significantly different between oral and parenteral antibiotic regimens (20 % versus 22 %, P = 0.68). Of the 953 episodes treated in the outpatient setting and 676 episodes treated with oral antibiotics, none were associated with infection-related mortality.
Conclusion
Based on the combination of results from all prospective studies to date, outpatient and oral antibiotic management of low-risk FN are effective in children and should be incorporated into clinical care where feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile neutropenia (FN) in patients with cancer is recognized as a common, potentially fatal complication of cytotoxic chemotherapy [7]. Hospitalization and prompt initiation of empiric broad-spectrum intravenous antibiotic therapy has dramatically reduced infection-related morbidity and mortality over the past 40 years [37, 40]. The intensity of this approach, however, is not without undesirable sequelae. Prolonged antibiotic exposure and hospitalization are potent risk factors for the emergence of resistant microorganisms [14] and secondary infection [20]. Inpatient therapy is also associated with inferior health-related quality of life for children with cancer [42]. Finally, hospitalization is significantly less cost-effective when compared to outpatient management of FN [44].
Children with FN are a heterogeneous group [38], and a subset at low-risk of adverse events might benefit from less intensive therapy [3]. Numerous prediction rules developed for risk stratification in pediatrics [36] have made it possible for the use of less intensive strategies in the management of low-risk patients. Two less-intensive strategies that have been employed in many studies are outpatient management and oral antibiotic administration. Outpatient management, using oral or parenteral antibiotics, can also include step-down management involving a short hospital admission followed by early discharge with home antibiotic therapy. Oral antibiotic regimens, in the inpatient or outpatient setting, can also include step-down management involving intravenous therapy for a short period followed by oral antibiotics.
It is generally accepted that adult inpatients with low-risk FN can be managed effectively with oral antibiotics [15, 22, 46], and there is increasing evidence that outpatient management of this population is an equally safe alternative [10]. As a result, risk-adapted international treatment guidelines have been established in adult oncology [16, 23]. However, despite several prospective studies addressing the question, there is no consensus on whether therapeutic intensity can be reduced safely in children with low-risk FN [4].
We recently conducted a systematic review of inpatient versus outpatient and oral versus parenteral antibiotic management of FN by analyzing randomized controlled trials (RCTs) and found no differences in efficacy or safety [45]. However, only two studies of outpatient management with 278 subjects were conducted in children, and one of these consisted of high-risk patients. Thus, estimates were highly unreliable and poorly generalizable to children with low-risk FN. We realized that more information was available from prospective single-arm studies which could supplement data from randomized trials, possibly resulting in more complete and robust information about the efficacy and safety of these strategies in pediatrics.
Our primary objective was to determine if there is a difference in efficacy between initial or step-down outpatient management and inpatient management of children with low-risk FN. Our secondary objective was to determine if there is a difference in efficacy between initial or step-down oral antibiotic therapy and parenteral therapy in children with low-risk FN.
Materials and methods
Data sources and searches
We developed a protocol for the review following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies [47]. We performed electronic searches of OVID Medline (1980 to March 7, 2011); EMBASE (1980 to March 7, 2011); and the Cochrane Central Register of Controlled Trials (until the first quarter of 2011). The search strategy (Appendix 1) included the Medical Subject Headings (MeSH) and text words “fever” and “neutropenia”, and was limited to studies conducted after 1980 and those published in the English language. All trial designs were included in the search.
Study selection
We defined inclusion and exclusion criteria a priori. Studies were included if: (1) the study examined any infection outcome of a homogeneous initial empiric regimen, (2) the population consisted of children or results were abstractable for the pediatric sub-group, and (3) the study was conducted prospectively (to avoid bias associated with retrospective studies). Exclusion criteria were: (1) conference proceeding only, (2) not published in English, (3) not a study, (4) retrospective, (5) population did not consist of children or data not abstractable for children, (6) cohort did not consist of patients with initial presentation of FN (i.e., enrolled ≥24 h after initial empiric treatment), (7) antibiotics studied were not initial empiric therapy, (8) heterogeneous empiric therapy regimens, (9) pharmacokinetic studies, (10) no infection outcomes reported, and (11) duplicate publications. Among this set of studies, those of low-risk patients only, as defined by each study protocol, were then selected.
One reviewer (LS) evaluated the titles and abstracts of publications identified by the search strategy, and any publication thought to be potentially relevant was retrieved in full. Two independent reviewers (AM and LS) then assessed full publications for eligibility; reviewers were not blinded to study authors or outcomes. Final inclusion of studies into the meta-analysis was by agreement of both reviewers. Agreement between reviewers was evaluated using a kappa statistic. Strength of agreement as evaluated by the kappa statistic was defined as slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00) [25].
Data extraction and quality assessment
Data extraction from included trials was performed independently by two reviewers (AM and LS) using a standardized data collection form.
The primary outcome measure for both objectives was treatment failure at 30 days when antibiotic modification was included as a criterion for failure. Although the trials used heterogeneous definitions of treatment failure, most definitions included persistence, recurrence or worsening of fever/infecting organisms, new infections, any modification of antibiotics, readmission, or death during study drug treatment. Secondary outcome measures were 30-day overall mortality and infection-related mortality, treatment failure when antibiotic modification was excluded from the failure definition, fever duration, recurrent infection (reappearance of infection or fever after initial resolution), sepsis, secondary infection (occurrence of new infection during treatment), adverse events leading to antibiotic discontinuation, and readmission to hospital. An outcome was presented only if each sub-group contained at least one study reporting that outcome.
Study quality was assessed using a modified version of an instrument previously developed to describe quality in studies of prognosis [18]. This quality assessment instrument examines four potential sources of bias: study participation, study attrition, confounding variables, and measurement of outcomes. Each element was rated as having low, medium, or high risk of bias for each study.
Data synthesis and analysis
This meta-analysis combined data at the study level and not at the individual patient level. All outcomes were described as percentages, with the exception of duration of fever which was described using the mean. For this outcome, we made the following assumptions to facilitate data synthesis: the mean can be approximated by the median and the range contains six standard deviations. Each study was weighted by the inverse variance. Given the anticipation of heterogeneity between studies, a random effects model [12] was used for all analyses. Because our outcomes were single percentages and not within-study comparisons, we did not test for publication bias as we did not believe it was relevant in this context.
The meta-analysis was performed using Review Manager (RevMan) (Version 5.1.0, The Cochrane Collaboration, Oxford, England). Agreement was calculated using the SAS statistical program (SAS-PC, version 9.1; SAS Institute Inc., Cary, NC). Sub-groups were defined based on treatment setting (outpatient or inpatient) and route of administration (oral or parenteral). A test for heterogeneity across subgroup results was used to determine if outcomes were modified based upon treatment setting or route of administration, with statistical significance defined as P < 0.10.
Results
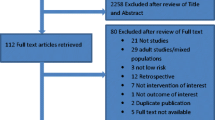
Figure 1 illustrates the flow diagram of trial identification and selection. A total of 7,281 titles and abstracts were reviewed, and 380 full articles were retrieved. Of these, 66 satisfied pre-defined inclusion criteria. Reasons for excluding 314 articles are provided in Fig. 1. The reviewers had almost perfect agreement on articles for inclusion (kappa = 0.98; 95 % CI 0.96, 1.00).
Of the 66 studies, 16 studies were restricted to low-risk patients [1, 5, 6, 9, 13, 17, 21, 24, 26, 28, 29, 31–34, 41] and were thus included in this analysis. For these 16 studies, the number of studies that demonstrated low risk of bias was as follows: 13 (81 %) for study participation, 14 (88 %) for study attrition, 2 (13 %) for confounding, and 7 (44 %) for measurement of outcomes.
Clinical characteristics of the 16 studies are presented in Table 1. Eight were RCTs and eight were prospective non-randomized studies. There were no RCTs that randomized the trial setting (outpatient versus inpatient). Five out of eight RCTs randomized oral versus parenteral antibiotics. The 16 studies described 24 treatment regimens and their outcomes. None of these regimens mandated the use of granulocyte colony-stimulating factor or antibiotic prophylaxis.
Outpatient vs. inpatient management
Table 2 compares outcomes of outpatient and inpatient management. Sixteen outpatient regimens, including three step-down regimens, were evaluated in 1,078 FN episodes while eight inpatient regimens were evaluated in 317 episodes. Nine out of 16 outpatient regimens and two out of eight inpatient regimens consisted of oral antibiotics. The following outcomes were not reported due to a lack of studies reporting these outcomes in either treatment setting: recurrent infection, sepsis, secondary infection, and readmission.
Treatment failure, when its definition included modification of antibiotics, was less likely to occur in the outpatient setting compared with the inpatient setting (15 % versus 28 %, P = 0.04) as demonstrated by the forest plot in Fig. 2. Overall and infection-related mortality were not significantly different between the inpatient and outpatient groups. There were two infection-related deaths in total; both patients had received inpatient therapy. Among 953 outpatient episodes, no infection-related deaths were reported. Treatment failure, when its definition excluded antibiotic modification, was not significantly different in both settings (Table 2).
Forest plot of treatment failure, including antibiotic modification, comparing outpatient with inpatient empiric regimens. Squares indicate percentages with horizontal lines representing 95 % CIs. Diamonds represent overall percentages from the meta-analysis with corresponding 95 % CIs. A test for heterogeneity [19] across subgroup results was used to determine if outcomes were modified based upon treatment setting or route of administration
Oral vs. parenteral antibiotics
Table 3 compares outcomes of oral and parenteral antibiotic management. Eleven oral regimens, including five step-down regimens, were evaluated in 785 FN episodes, while 13 parenteral regimens were evaluated in 610 episodes. Nine out of 11 oral regimens and 7 out of 13 parenteral regimens were delivered in the outpatient setting. Oral antibiotic regimens consisted of: fluoroquinolone monotherapy (7 regimens, 581 episodes), fluoroquinolone and amoxicillin-clavulanate (3 regimens, 159 episodes), and cefixime (1 regimen, 45 episodes). Of the 10 studies using fluoroquinolones, 6 used ciprofloxacin (332 episodes), 3 used ofloxacin (207 episodes), and 1 used gatifloxacin (201 episodes).
Treatment failure, when its definition included modification of antibiotics, was not significantly different between oral and parenteral antibiotic regimens (20 % versus 22 %, P = 0.68) as demonstrated by the forest plot in Fig. 3. There were two infection-related deaths in total; both had been treated with parenteral antibiotics. Among 676 episodes treated with oral regimens, no infection-related deaths were reported. There was no significant increase in treatment failure excluding antibiotic modification, duration of fever, recurrent infection, sepsis, secondary infection, adverse events, or readmission associated with oral therapy.
Forest plot of treatment failure, including antibiotic modification, comparing oral with parenteral empiric regimens. Squares indicate percentages with horizontal lines representing 95 % CIs. Diamonds represent overall percentages from the meta-analysis with corresponding 95 % CIs. A test for heterogeneity [19] across subgroup results was used to determine if outcomes were modified based upon treatment setting or route of administration
Discussion
When the results of all prospective studies are combined, we were unable to demonstrate any difference in the efficacy of outpatient and oral antibiotic therapy compared with inpatient and parenteral antibiotic therapy, respectively. In particular, among all pediatric published studies of low-risk pediatric FN, 0 out of 953 episodes treated in the outpatient setting and 0 out of 676 episodes treated with oral antibiotics were associated with infection-related mortality, thereby underscoring the potential safety of these regimens in low-risk children.
Using our inclusion criteria, no trials were identified that randomized inpatient versus outpatient therapy. However, by combining data by regimen, we were able to calculate and compare outcomes between inpatient and outpatient protocols. Interestingly, treatment failure, including therapy modification, was more likely in the inpatient group. Since there was no difference in treatment failure between the two groups when antibiotic modification was excluded as a cause, it is likely that this difference was related to more frequent modification of therapy in the inpatient group. It is possible that inpatients were more likely to develop sepsis or hospital-acquired secondary infection which responded to a change in therapy. It is also possible that with closer clinical follow-up, modifications were more likely to occur in inpatients with persistent fever or suspected minor infections compared with outpatients, resulting in more frequent and potentially unnecessary antibiotic modification. However, we could not examine this in our meta-analysis as no inpatient studies reported these outcomes.
The secondary objective of this study was to evaluate whether the route of drug administration has an impact on the outcome of low-risk pediatric FN. We did not find a significant difference in treatment failure rates or infection-related mortality between oral and parenteral management. These findings are consistent with RCTs comparing oral and parenteral regimens in children [9, 17, 28, 31, 34]. When antibiotic modification was included as a cause of failure, treatment failure rates were about 20 % in both oral and parenteral groups.
Our results are important because although it is recognized that adult low-risk FN may be managed with outpatient or oral antibiotics as reflected by international treatment guidelines [16, 23], there is much more uncertainty in children. Initial management of low-risk pediatric FN in many oncology centers continues to involve inpatient parenteral antibiotics [8, 11, 35]. Previous evidence of the efficacy of outpatient or oral antibiotic therapy in this population has been limited by the very small number of randomized trials conducted in pediatrics, which has led to caution in extrapolating results to this population. However, our results, which are the most comprehensive to date, verify the efficacy of these approaches in pediatric patients. These good outcomes may be expected since at least one study has demonstrated that a highly selected group of adult and pediatric low-risk patients may be managed without antibiotics [30].
We limited eligibility to those studies which enrolled children within 24 h of FN presentation and to low-risk patients. We chose to restrict our analysis to those with new-onset FN as studies that enroll patients 24 h or more after FN onset might exclude patients who experience adverse outcomes early in the episode. We also limited the analysis to studies of low-risk patients as we believe that lower intensity therapy is most likely to be acceptable in this patient population in high-income countries. This approach resulted in the exclusion of two randomized studies [2, 39]. However, it is noteworthy that both studies showed similar results to ours.
One limitation of our analysis was that we relied on each study protocol’s definition of low-risk criteria. Risk in FN is likely to be a continuous variable [23], and as a result, the risk status of patients may have varied from trial to trial. Consequently, despite restricting the analysis to “low-risk” patients, it is possible that the outcomes in our comparison of treatment setting and route of administration may still be confounded by risk status. Nonetheless, in spite of this heterogeneity, we demonstrated no infection-related deaths in the outpatient and oral sub-groups irrespective of variable definitions of low-risk FN. Another limitation of this meta-analysis was the variability in definition of our primary outcome, treatment failure, across all studies. This composite endpoint encompassed multiple outcomes in most of the evaluated studies, resulting in significant heterogeneity. Furthermore, because treatment failure included readmission in most studies, outpatient failure rates may have been overestimated in our meta-analysis as readmission pertains only to outpatients. Finally, we restricted our review to studies published in the English language. However, a previous review found that restriction of systematic reviews to English, when compared to the inclusion of other languages, does not bias results [27].
In this study, we failed to demonstrate significant differences between sub-groups based upon tests of heterogeneity. It is possible that this failure to demonstrate significant heterogeneity was due to inadequate power; this is especially important for comparisons of outcome variables such as mortality in which the number of observed events was very low.
Our meta-analysis suggests that initial and step-down outpatient or oral antibiotic management of low-risk FN are effective in children. Centers should therefore adopt these lower-intensity strategies for low-risk pediatric FN patients if the infrastructure to safely manage outpatients can be established. While useful for clinical decision-making, the results of this study may also assist in educating and reassuring patients and families who might otherwise prefer inpatient management of FN [43]. Future research should focus on knowledge translation and effectiveness analysis of large numbers of patients treated outside of research protocols as more centers begin to adopt these lower-intensity strategies for low-risk pediatric FN.
References
Abbas AAH, Felimban SK, Cittana BA et al (2003) Once daily ceftriaxone and amikacin for outpatient treatment of neutropenic fever in children with acute lymphoblastic leukaemia. Haema 6(4):501–506
Ahmed N, El-Mahallawy HA, Ahmed IA, Nassif S, El-Beshlawy A, El-Haddad A (2007) Early hospital discharge versus continued hospitalization in febrile pediatric cancer patients with prolonged neutropenia: a randomized, prospective study. Pediatr Blood Cancer 49:786–792
Ammann RA, Bodmer N, Hirt A et al (2010) Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol 28:2008–2014
Ammann RA, Simon A, de Bont ES (2005) Low risk episodes of fever and neutropenia in pediatric oncology: is outpatient oral antibiotic therapy the new gold standard of care? Pediatr Blood Cancer 45:244–247
Aquino VM, Herrera L, Sandler ES, Buchanan GR (2000) Feasibility of oral ciprofloxacin for the outpatient management of febrile neutropenia in selected children with cancer. Cancer 88:1710–1714
Bartolozzi S, Clerico A, Properzi E, Minori A, Castello MA (1997) Ceftriaxone as a single agent in empirical therapy of unexplained fever in granulocytopenic children with solid tumors. J Chemother 9:227–231
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328–340
Boragina M, Patel H, Reiter S, Dougherty G (2007) Management of febrile neutropenia in pediatric oncology patients: a Canadian survey. Pediatr Blood Cancer 48:521–526
Cagol AR, Castro Junior CG, Martins MC et al (2009) Oral vs. intravenous empirical antimicrobial therapy in febrile neutropenic patients receiving childhood cancer chemotherapy. J Pediatr (Rio J) 85:531–535
Carstensen M, Sorensen JB (2008) Outpatient management of febrile neutropenia: time to revise the present treatment strategy. J Support Oncol 6:199–208
Chamberlain JD, Smibert E, Skeen J, Alvaro F (2005) Prospective audit of treatment of paediatric febrile neutropenia in Australasia. J Paediatr Child Health 41:598–603
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Duzova A, Kutluk T, Kanra G et al (2001) Monotherapy with meropenem versus combination therapy with piperacillin plus amikacin as empiric therapy for neutropenic fever in children with lymphoma and solid tumors. Turk J Pediatr 43:105–109
El-Mahallawy HA, El-Wakil M, Moneer MM, Shalaby L (2011) Antibiotic resistance is associated with longer bacteremic episodes and worse outcome in febrile neutropenic children with cancer. Pediatr Blood Cancer 57:283–288
Freifeld A, Marchigiani D, Walsh T et al (1999) A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med 341:305–311
Freifeld AG, Bow EJ, Sepkowitz KA et al (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93
Gupta A, Swaroop C, Agarwala S, Pandey RM, Bakhshi S (2009) Randomized controlled trial comparing oral amoxicillin-clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low-risk febrile neutropenia. J Pediatr Hematol Oncol 31:635–641
Hayden JA, Cote P, Bombardier C (2006) Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144:427–437
Higgins JPT, Green S, Cochrane Collaboration (2008) Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell, Chichester; Hoboken
Kamboj M, Sepkowitz KA (2009) Nosocomial infections in patients with cancer. Lancet Oncol 10:589–597
Kaplinsky C, Drucker M, Goshen J, Tamary H, Cohen IJ, Zaizov R (1994) Ambulatory treatment with ceftriaxone in febrile neutropenic children. Isr J Med Sci 30:649–651
Kern WV, Cometta A, De Bock R, Langenaeken J, Paesmans M, Gaya H (1999) Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med 341:312–318
Klastersky J, Paesmans M, Rubenstein EB et al (2000) The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18:3038–3051
Kutluk T, Kurne O, Akyuz C et al (2004) Cefepime vs. meropenem as empirical therapy for neutropenic fever in children with lymphoma and solid tumours. Pediatr Blood Cancer 42:284–286
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Malik IA (1997) Out-patient management of febrile neutropenia in indigent paediatric patients. Ann Acad Med Singap 26:742–746
Moher D, Pham B, Klassen TP et al (2000) What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol 53:964–972
Mullen CA, Petropoulos D, Roberts WM et al (1999) Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 86:126–134
Mustafa MM, Aquino VM, Pappo A, Tkaczewski I, Buchanan GR (1996) A pilot study of outpatient management of febrile neutropenic children with cancer at low risk of bacteremia. J Pediatr 128:847–849
Oude Nijhuis C, Kamps WA, Daenen SM et al (2005) Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J Clin Oncol 23:7437–7444
Paganini H, Gomez S, Ruvinsky S et al (2003) Outpatient, sequential, parenteral-oral antibiotic therapy for lower risk febrile neutropenia in children with malignant disease: a single-center, randomized, controlled trial in Argentina. Cancer 97:1775–1780
Paganini H, Rodriguez-Brieshcke T, Zubizarreta P et al (2001) Oral ciprofloxacin in the management of children with cancer with lower risk febrile neutropenia. Cancer 91:1563–1567
Petrilli A, Altruda Carlesse F, Alberto Pires Pereira C (2007) Oral gatifloxacin in the outpatient treatment of children with cancer fever and neutropenia. Pediatr Blood Cancer 49:682–686
Petrilli AS, Dantas LS, Campos MC, Tanaka C, Ginani VC, Seber A (2000) Oral ciprofloxacin vs. intravenous ceftriaxone administered in an outpatient setting for fever and neutropenia in low-risk pediatric oncology patients: randomized prospective trial. Med Pediatr Oncol 34:87–91
Phillips B, Selwood K, Lane SM, Skinner R, Gibson F, Chisholm JC (2007) Variation in policies for the management of febrile neutropenia in United Kingdom Children’s Cancer Study Group centres. Arch Dis Child 92:495–498
Phillips B, Wade R, Stewart LA, Sutton AJ (2010) Systematic review and meta-analysis of the discriminatory performance of risk prediction rules in febrile neutropaenic episodes in children and young people. Eur J Cancer 46:2950–2964
Pizzo PA (1993) Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med 328:1323–1332
Rondinelli PI, Ribeiro Kde C, de Camargo B (2006) A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol 28:665–670
Santolaya ME, Alvarez AM, Aviles CL et al (2004) Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. J Clin Oncol 22:3784–3789
Schimpff S, Satterlee W, Young VM, Serpick A (1971) Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med 284:1061–1065
Shrestha PN, Sah KP, Rana R (2009) Emperical oral antibiotic therapy for children with low risk febrile neutropenia during cancer chemotherapy. J Nepal Paediatr Soc 29(1):22–25
Speyer E, Herbinet A, Vuillemin A, Chastagner P, Briancon S (2009) Agreement between children with cancer and their parents in reporting the child’s health-related quality of life during a stay at the hospital and at home. Child Care Health Dev 35(4):489–495
Sung L, Feldman BM, Schwamborn G et al (2004) Inpatient versus outpatient management of low-risk pediatric febrile neutropenia: measuring parents’ and healthcare professionals’ preferences. J Clin Oncol 22:3922–3929
Teuffel O, Amir E, Alibhai SM, Beyene J, Sung L (2011) Cost-effectiveness of outpatient management for febrile neutropenia in children with cancer. Pediatrics 127:e279–e286
Teuffel O, Ethier MC, Alibhai SM, Beyene J, Sung L (2011) Outpatient management of cancer patients with febrile neutropenia: a systematic review and meta-analysis. Ann Oncol 11:2358–2365
Vidal L, Paul M, Ben-Dor I, Pokroy E, Soares-Weiser K, Leibovici L (2004) Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst Rev: CD003992
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Acknowledgements
We would like to thank Elizabeth Uleryk for her gracious assistance and expertise in conduct of the literature search. We also would like to acknowledge Rhonda Adams for her administrative assistance.
LS is supported by a New Investigator Award with the Canadian Institutes of Health Research.
Conflict of interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Search strategies used to identify eligible studies
Ovid MEDLINE(R)
-
1
agranulocytosis/or neutropenia/or leukopenia/
-
2
fever/ or “fever of unknown origin”/
-
3
1 and 2
-
4
(febrile adj5 (neutropen* or granulocytop* or agranulocyto* or leukocytop??ni*)).ti,ab.
-
5
3 or 4
-
6
(“clinical trial, all” or clinical trial).pt. or clinical trials as topic/
-
7
clinical trial, phase i.pt. or clinical trials, phase i as topic/
-
8
clinical trial, phase ii.pt. or clinical trials, phase ii as topic/
-
9
clinical trial, phase iii.pt. or clinical trials, phase iii as topic/
-
10
clinical trial, phase iv.pt. or clinical trials, phase iv as topic/
-
11
controlled clinical trial.pt. or controlled clinical trials as topic/
-
12
meta-analysis.pt. or meta-analysis as topic/
-
13
multicenter study.pt. or multicenter studies as topic/
-
14
randomized controlled trial.pt. or randomized controlled trials as topic/
-
15
or/6–14
-
16
cohort studies/ or longitudinal studies/or follow-up studies/or prospective studies/
-
17
case–control studies/or retrospective studies/or cross-sectional studies/
-
18
prognosis/ or disease-free survival/or medical futility/or pregnancy outcome/or treatment outcome/or treatment failure/
-
19
disease progression/
-
20
morbidity/or incidence/or prevalence/
-
21
mortality/or cause of death/or fatal outcome/or hospital mortality/or infant mortality/or maternal mortality/or survival rate/
-
22
survival analysis/or disease-free survival/
-
23
natural histor???.tw.
-
24
predictive value of tests/
-
25
or/16–24
-
26
15 or 25
-
27
5 and 26
-
28
limit 27 to yr = “1980–Current”
-
29
limit 28 to English language
EMBASE
-
1
leukopenia/or agranulocytosis/or neutropenia/
-
2
pyrexia idiopathica/or fever/or (fever* adj5 unknown adj5 origin*).ti,ab.
-
3
1 and 2
-
4
febrile neutropenia/
-
5
3 or 4
-
6
ct.fs. or clinical trial/or phase 1 clinical trial/or phase 2 clinical trial/or phase 3 clinical trial/or phase 4 clinical trial/or controlled clinical trial/or randomized controlled trial/or multicenter study/or meta analysis/or double-blind procedure/or single blind procedure/or triple blind procedure/or (random* or rct or rcts or ((singl: or doubl: or tripl: or trebl:) and (mask: or blind:))).mp.
-
7
5 and 6
-
8
cohort analysis/or longitudinal study/or prospective study/or case–control study/or hospital based case–control study/or population based case–control study/or retrospective study/or cancer recurrence/or cancer regression/or cancer relapse/or disease duration/or disease exacerbation/or prognosis/or recurrent disease/or reinfection/or relapse/or remission/or tumor recurrence/or tumor regression/or survival/or cancer survival/or disease free survival/or overall survival/or survival rate/or survival time/or incidence/or cancer incidence/or familial incidence/or morbidity/or maternal morbidity/or perinatal morbidity/or newborn morbidity/or mortality/or cancer mortality/or childhood mortality/or embryo mortality/or fetus mortality/or infant mortality/or maternal mortality/or prenatal mortality/or surgical mortality/or perinatal mortality/or newborn mortality/or death/or “cause of death”/or dying/or heart death/or sudden death/or child death/or newborn death/or prevalence/or treatment outcome/or disease free interval/or treatment failure/or drug treatment failure/or (natural adj2 history).mp. (2419301)
-
9
5 and 8
-
10
7 or 9
-
11
4 and 6
-
12
4 and 8
-
13
11 or 12
-
14
limit 13 to English language
Cochrane Central Register of Controlled Trials
-
1
agranulocytosis/ or neutropenia/ or leukopenia/
-
2
fever/ or "fever of unknown origin"/
-
3
1 and 2
-
4
(febrile adj5 (neutropen* or granulocytop* or agranulocyto* or leukocytop??ni*)).ti,ab.
-
5
3 or 4 (1020)
-
6
limit 5 to yr = “1980–Current”
Rights and permissions
About this article
Cite this article
Manji, A., Beyene, J., Dupuis, L.L. et al. Outpatient and oral antibiotic management of low-risk febrile neutropenia are effective in children—a systematic review of prospective trials. Support Care Cancer 20, 1135–1145 (2012). https://doi.org/10.1007/s00520-012-1425-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1425-8