Abstract
Severe oral mucositis developed in allogeneic hematopoietic stem cell transplantation (HSCT) accompanies intolerable pain and risk for systemic bacteremia infection. Conventional stem cell transplantation (CST) and reduced-intensity regimens for allogeneic HSCT (RIST) may differently affect the occurrence and severity of oral mucositis. Here, we comparatively examined oral mucositis in patients undergoing CST and that in RIST patients to search for measures to alleviate oral mucositis. We retrospectively analyzed the data of 130 consecutive patients undergoing HSCT (conventional, 60; RIST, 70). Oral mucositis was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. We also investigated the risk factors for severe oral mucositis in each regimen. The incidence of oral mucositis was not significantly different between RIST and CST patients. The use of opioid analgesics to control pain due to oral mucositis was significantly less in patients undergoing RIST compared with those receiving CST. The risk factors for severe oral mucositis, determined by univariate and multivariate analyses, were “younger age (<40)” in CST and “longer duration of neutropenia (≥14 days)” in RIST. Although the incidences of oral mucositis were almost the same, the need for opioid analgesics and the risk factors for severe oral mucositis differed between CST and RIST patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis is one of the most common complications associated with allogeneic hematopoietic stem cell transplantation (HSCT). It was seen in 60–90% of patients who had received stem cell transplantation [1–3]. The oral mucositis in HSCT accompanies so severe pain that it can lead to anorexia and dehydration, and a large population of patients with severe oral mucositis requires total parenteral nutrition and opioid analgesics [4]. Severe oral mucositis is also associated with worse clinical and economic outcomes, especially systemic bacteremia infection [5].
Recently, reduced-intensity conditioning regimens for allogeneic HSCT (RIST) have been developed for patients who are considered unsuitable for conventional stem cell transplantation (CST) because of advanced age or medical contraindications [6, 7]. The conditioning regimens typically include a purine analog, such as fludarabine (FLU), an alkylating agent, or low-dose total body irradiation (TBI). We need to consider the differences between CST and RIST protocols in the effects on oral mucositis because such a variety of RIST protocols have been developed and their toxicity profiles can make differences in the degree of immunosuppression or myeloablation [2, 3, 8–10].
The present study was a retrospective analysis to compare oral mucositis in 70 consecutive patients who had received RIST, which mainly consisted of FLU, busulfan (BU), and TBI, with that in 60 patients who had received CST during the same period. We also investigated risk factors for severe oral mucositis in each regimen.
Materials and methods
Patients
We retrospectively analyzed the data of 130 consecutive patients undergoing HSCT between March 2006 and December 2009 at Stem Cell Transplantation Center of Hokkaido University Hospital (M, 67; F, 63; 47.6 ± 15.2 years). CST and RIST were administered to 60 (M, 28; F, 32) and 70 (M, 39; F, 31) patients, respectively. Characteristics of the patients and transplantation are shown in Table 1. The ethical committee of Hokkaido University Hospital approved this study. An informed consent was obtained from each patient.
Conditioning regimens
Most of the conventional conditioning regimens consisted of TBI (12 Gy in six fractions) plus cyclophosphamide (60 mg/kg once daily i.v. for 2 days, total dose of 120 mg/kg) ± VP-16 (15 mg/kg once daily i.v. for 2 days, total dose of 30 mg/kg) [11, 12], and most of the reduced-intensity conditioning regimens consisted of FLU (30 mg/m2 once daily i.v. for 6 days, total dose of 180 mg/m2) plus oral BU (4 mg/kg p.o. in divided doses daily for 2 days, total dose of 8 mg/kg) or intravenous BU (3.2 mg/kg i.v. in divided doses daily for 2 days, total dose of 6.4 mg/kg) plus low-dose TBI (4 Gy in two fractions). Cyclosporine A (CsA, 3 mg/kg) or tacrolimus (FK, 0.03 mg/kg) and short-course methotrexate (MTX) were used for graft-versus-host disease (GVHD) prophylaxis. MTX was given at a dose of 15 mg/m2 or 10 mg/m2 on day 1, and 10 mg/m2 or 7 mg/m2 on day 3 and day 6.
Assessment of oral mucositis
Oral mucositis was graded as follows according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0[13]:
-
Grade 1:
Erythema of the mucosa
-
Grade 2:
Patchy ulcerations or pseudomembranes
-
Grade 3:
Confluent ulcerations or pseudomembranes, bleeding in response to minor trauma
-
Grade 4:
Tissue necrosis, significant spontaneous bleeding, life-threatening consequences
-
Grade 5:
Death
Grading was done daily by nurses under the instruction of dentists, and the consistency of assessments was double-checked by the dentists during their rounds at least once per week. Severe oral mucositis was defined as grades 3–4.
Assessment of use of opioid analgesics to control pain due to oral mucositis
The use of opioid analgesics to control pain due to oral mucositis was evaluated for all patients, and frequencies of its use were compared among HSCT types.
Oral management
All subjects were referred to dentists, and necessary dental treatment was completed before HSCT. Namely, at least two dentists examined the patients’ oral health, including oral hygiene and potential causes of infections in the oral region by radiographic survey and by clinical examination of the hard and soft tissues and dental problems that might cause infection, such as periapical and marginal periodontitis, dental caries, and semi-impacted or impacted teeth, were treated by surgical procedures as much as possible until HSCT. All subjects received instruction regarding self-management of oral hygiene: tooth brushing after every meal and before going to bed, and oral rinsing with normal saline solution every 3 h during the day. The dentists and hygienists weekly performed an oral examination on the patients and monitored their compliance in a clean room.
Statistical analysis
Univariate analyses were performed using the chi-square test and Fisher’s exact test, as appropriate. The factors with a P value of 0.05 or less in the univariate analyses were included in the multivariate analysis. Multivariate logistic regression models were used to analyze the influence of selected variables on the risk for severe oral mucositis. For most of the statistical analysis, SPSS 14.0 for Windows (SPSS, Chicago, IL, USA) was used. The P value was set to <0.05 as significant.
Results
Patients and transplantation characteristics
Characteristics of the patients and transplantations are shown in Table 1. Median age, underlying disease, and disease status at transplantation were significantly different between CST and RIST patients. Other parameters such as sex, TBI, and GVHD prophylaxis were not different between CST patients and RIST patients.
Incidences and severity of oral mucositis in CST and RIST
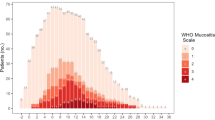
As shown in Table 2, the incidences of oral mucositis (>grade 1) were not significantly different between CST and RIST patients according to the NCI-CTCAE; the frequencies were 83.3% (50/60) and 75.7% (53/70), respectively. Severe mucositis (grades 3 and 4) was observed in 33.3% (20/60) of CST patients and 32.9% (23/70) of RIST patients, which showed no significant difference. However, a significantly lower percentage of patients undergoing RIST (32.2%) required opioid analgesics to control pain due to oral mucositis compared with those undergoing CST (60.4%) as shown in Fig. 1 (P = 0.0028).
Univariate and multivariate analyses for severe oral mucositis in CST and RIST
To identify the risk factors for severe mucositis in CST and RIST, a univariate and multivariate analyses were performed in each regimen. The results in CST are summarized in Table 3. The univariate analysis showed that “younger age (<40)”, “VP-16 regimen”, and “longer duration of neutropenia (≥14 days)” were significantly associated with a high incidence of severe oral mucositis in CST. Of those, only “younger age (<40)” remained significant in multivariate analysis (odds ratio, 5.6; 95%CI, 1.9–16.5; P < 0.05). With regards to RIST, the results are summarized in Table 4. Only “longer duration of neutropenia (≥14 days)” was significantly associated with sever oral mucositis in RIST in both univariate and multivariate analyses (odds ratio, 12.4; 95%CI, 1.4–109; P = 0.02).
Discussion
The results of this study are summarized as follows: (1) The incidence of oral mucositis was almost the same between CST and RIST patients; (2) The use of opioid analgesics to control pain due to oral mucositis was significantly less in patients undergoing RIST compared with those receiving CST; (3) Univariate and multivariate analyses revealed that the risk factors for severe oral mucositis were “younger age (<40)” in CST and “longer duration of neutropenia (≥14 days)” in RIST.
While Takahashi et al. reported that the severity of oral mucositis was reduced in RIST patients compared with CST patients [1], no significant difference was observed in the incidence of severe oral mucositis between patients who received CST and those who received RIST in our study. Several studies reported that severe oral mucositis was correlated with TBI [14, 15]. One of the reasons for this “no significant difference” in our study might be associated with the use of TBI in most RIST patients. The patients who received our RIST regimen including TBI tended to have a longer neutropenic period and more mucosal injury than those in patients who received other RIST regimens [16, 17]. Furthermore, both CST and RIST regimens in the present cases used the same doses of MTX on days 1, 3, and 6 as GVHD prophylaxis.
Severe oral mucositis causes intolerable pain, which is often controlled by the administration of opioid analgesics. As recent trends in cancer pain control recommend the appropriate use of narcotics to minimize pain, the use of opioid analgesics in RIST patients was significantly less compared with that in CST patients. As RIST tends to dispense with narcotics, their major side effects such as ileus could be also avoided.
In multivariate analysis, “younger age (<40)” was significantly associated with severe oral mucositis in CIST patients (odds ratio, 5.6; 95%CI, 1.9–16.5; P < 0.05). This confirms the report of Vagliano where severe oral mucositis was observed more in adult patients than in the elderly patients [18]. Sonis reported that young patients, who typically have a higher proliferating fraction of basal cells, are three times more likely to develop mucositis than elderly adults in whom the basal cell proliferation is slow [19]. In RIST patients, the “duration of neutropenia (more than 14 days)” was significantly associated with severe oral mucositis in multivariate analysis (OR = 12.4, 95%CI 1.4–109, P = 0.024). Once patients developed oral mucositis, it continued to worsen during neutropenia. In those patients, it is important to prevent the development of oral mucositis.
Although our analysis has limitations due to its retrospective nature and the small sample size, our results showed that the need for opioid analgesics and the risk factors for severe oral mucositis differed between CST and RIST patients. Further prospective controlled studies are needed to assess the differences between CST and RIST for better management of oral mucositis in HSCT patients.
References
Takahashi K, Soga Y, Murayama Y, Udagawa M, Nishimoto H, Sugiura Y, Maeda Y, Tanimoto M, Takashiba S (2010) Oral mucositis in patients receiving reduced-intensity regimens for allogeneic hematopoietic cell transplantation: comparison with conventional regimen. Support Care Canc 18:115–119
Vokurka S, Steinerova K, Karas M, Koza V (2009) Characteristics and risk factors of oral mucositis after allogeneic stem cell transplantation with FLU/MEL conditioning regimen in context with BU/CY2. Bone Marrow Transplant 44:601–605
Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G et al (2008) Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant 42:275–279
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19:2201–2005
Giralt S, Estey E, Albitar M et al (1997) Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 89:4531–4536
Slavin S, Nagler A, Naparstek E et al (1998) Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91:756–763
Mohty M, Faucher C, Vey N et al (2000) High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant 26:251–255
Mohty M, Jacot W, Faucher C et al (2003) Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia 17:2168–2177
Toubai T, Tanaka J, Mori A et al (2004) Efficacy of etoposide, cyclophosphamide, and total body irradiation in allogeneic bone marrow transplantation for adult patients with hematological malignancies. Clin Transplant 18:552–557
Shigematsu A, Yamamoto S, Sugita J, Kondo T et al (2010) Increased risk of bacterial infection after engraftment in patients treated with allogeneic bone marrow transplantation following reduced-intensity conditioning regimen. Transpl Infect Dis 12(5):412–420
Shigematsu A, Kondo T, Yamamoto S et al (2008) Excellent outcome of allogeneic hematopoietic stem cell transplantation using a conditioning regimen with medium-dose VP-16, cyclophosphamide and total-body irradiation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 14:568–575
U.S. National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Gori E, Arpinati M, Bonifazi F, Errico A, Mega A, Alberani F et al (2007) Cryotherapy in the prevention of oral mucositis in patients receiving low-dose methotrexate following myeloablative allogeneic stem cell transplantation: a prospective randomized study of the Gruppo Italiano Trapianto di Midollo Osseo nurses group. Bone Marrow Transplant 39:347–352
Robien K, Schubert M, Bruemmer B, Lloid M, Potter J, Ulrich C (2004) Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J Clin Oncol 22:1268
Junghanss C, Marr KA, Carter RA et al (2002) Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant 8:512–525
Hori A, Kami M, Kim SW et al (2004) Development of early neutropenic fever, with or without bacterial infection, is still a significant complication after reduced-intensity stem cell transplantation. Biol Blood Marrow Transplant 10:65–72
Vagliano L, Feraut C et al. (2011) Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT (HSCT)–results of a multicentre study. Bone Marrow Transplant (in press)
Sonis ST (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34:39–43
Acknowledgments
We thank Ms. M. Yanome for her help in preparing the manuscript.
Financial disclosure
This work was supported in part by Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashiwazaki, H., Matsushita, T., Sugita, J. et al. A comparison of oral mucositis in allogeneic hematopoietic stem cell transplantation between conventional and reduced-intensity regimens. Support Care Cancer 20, 933–939 (2012). https://doi.org/10.1007/s00520-011-1164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1164-2