Abstract
Goals of work
The goal of this study was to develop a new, objective prognostic score (OPS) for terminally ill cancer patients based on an integrated model that includes novel objective prognostic factors.
Materials and methods
A multicenter study of 209 terminally ill cancer patients from six training hospitals in Korea were prospectively followed until death. The Cox proportional hazard model was used to adjust for the influence of clinical and laboratory variables on survival time. The OPS was calculated from the sum of partial scores obtained from seven significant predictors determined by the final model. The partial score was based on the hazard ratio of each predictor. The accuracy of the OPS was evaluated.
Main results
The overall median survival was 26 days. On the multivariate analysis, reduced oral intake, resting dyspnea, low performance status, leukocytosis, elevated bilirubin, elevated creatinine, and elevated lactate dehydrogenase (LDH) were identified as poor prognostic factors. The range of OPS was from 0.0 to 7.0. For the above cutoff point of 3.0, the 3-week prediction sensitivity was 74.7%, the specificity was 76.5%, and the overall accuracy was 75.5%.
Conclusions
We developed the new OPS, without clinician’s survival estimates but including a new prognostic factor (LDH). This new instrument demonstrated accurate prediction of the 3-week survival. The OPS had acceptable accuracy in this study population (training set). Further validation is required on an independent population (testing set).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survival prediction is an essential consideration for palliative care. The patient survival potential is often used to estimate the risks and benefits of therapies. Therefore, the clinical prediction of survival (CPS) is a key parameter for the management of end-of-life issues [16]. One of the most frequently asked questions of patients with advanced cancer is “How long do I have?” Although this question is common among patients and their families, an accurate CPS is difficult to determine, and most doctors find such a prediction extremely difficult [3, 17]. The uncertainty and complexities of terminal phase disease, the lack of education and training for assessing prognosis at this time [9], and the limited availability of objective parameters to determine such predictions add to the difficulties. The palliative prognostic (PaP) score is the most widely used validated prognostic score; it includes the CPS [21]. Although the CPS has unique validity [8] and its use in conjunction with other prognostic factors is highly recommended [18], it is in general not a familiar tool for inexperienced clinicians. The CPS is a subjective assessment and can vary with the clinician administering it; it commonly overestimates the length of actual survival [4, 8]. Most patients with advanced cancer experience a short phase of accelerated decline in the last month or two of life [6], and the accuracy of the CPS is especially limited during this terminal phase [10].
Some prognostic scores have improved the accuracy of the CPS. However, objective prognostic factors are needed that are reproducible, systematically applied, easily interpreted, and can be widely applied. Biological parameters could be included among the objective factors, such as an elevated lactate dehydrogenase (LDH) and C reactive protein (CRP) [6, 10, 18, 25, 26]. Therefore, the goal of this study was to develop an objective prognostic score (OPS) that does not depend on the CPS and include novel objective prognostic factors. A prospective, multicenter study was carried out to develop a new OPS for terminally ill cancer patients.
Materials and methods
Eleven physicians that belong to the Korean Palliative Medicine Research Group performed this study. The physicians are experienced family practice clinicians and have worked for more than 6 years in the field of palliative care. We followed terminal cancer inpatients until their death between November 2006 and December 2007. A total of 209 patients from six teaching hospitals were included in the study cohort. The inclusion criteria were as follows: (1) incurable cancer in the terminal stage confirmed by more than two physicians and inpatients for hospice care; (2) an age more than 18 years; (3) consented to participate in this study; and (4) the CPS was less than 3 months. The exclusion criteria were (1) chemotherapy or radiotherapy planned to actively treat the cancer and (2) hematologic malignancies or renal failure due to possible interference with some of the serological variables. Informed written consent for the use of personal information was provided by all participants. The study protocol was approved by the Institutional Review Board of the Korea University Guro Hospital.
Data were gathered at the time of hospitalization: demographic data (age and gender) and data related to the disease (primary cancer site, metastasis site, cancer treatment, and regular opioid usage). The clinical symptoms investigated by a staff physician were anorexia, dyspnea, and cognitive impairment. Anorexia was defined as less than five spoons per meal (about one-third amount of routine Korean main dish and rice); dyspnea was determined by the following question: “In the past 24 h, have you been short of breath at rest?” Cognitive impairment was assessed by a physician based on direct observation on the day of the study as “present” or “absent.” Pain severity was estimated using a numeric rating scale (NRS; range, 0–10).
The performance status was measured using the Eastern Cooperative Oncology Group (ECOG; range, 0–4) performance status scale. Routine laboratory tests were performed on admission. The parameters recorded were the white blood cell count, total bilirubin, creatinine, CRP, LDH, and uric acid. Survival time was defined as the period from the day of admission to the day of death in the hospital. The research was concluded on December 31, 2007.
Statistical analysis
Serum LDH, CRP, and uric acid levels were categorized into two groups based on the 50th percentile. The serum bilirubin and creatinine levels were divided by abnormal values for hepatic and renal dysfunction based on the methods reported in a previous study [24], respectively. Leukocytosis was defined as cell counts of over 11,000 cells/mm3 of the total white blood cell count, as suggested by prior studies [21].
The median survival time of the subjects was determined by the Kaplan–Meier method. The log-rank test was performed to compare the survival time according to independent variables. Multivariate analysis of the relationships among variables and survival time was performed using Cox’s proportional hazard model. We examined the plot of ln{−ln[S(t)]}, where S(t) is the Kaplan–Meier estimate of the survival curves, against the logarithm of the time for each level of the variables evaluated in the study. The results suggested that the Cox regression model was the most appropriate for parametric modeling of the data. Hence, the final model was constructed using the Cox regression model fitted with a backward stepwise selection procedure. All of the variables were dichotomized to assess the hazard ratio (HR) in the multivariate analysis.
To establish a new OPS for the terminally ill cancer patients, the HRs for significant prognostic factors were used in the multivariate analysis. Anorexia (less than or equal to five spoons per meal: ≤1/3 amount of routine meal) and resting dyspnea were dichotomized as absent (no) or present (yes). The ECOG performance status was categorized as 1–3 and 4. The serum bilirubin levels were categorized as >2.0 and ≤ 2.0 mg/dL. The serum creatinine levels were categorized as ≥1.5 and <1.5 mg/dL. The serum LDH levels were categorized as ≥502 and <502 IU/L.
To assign a partial score value, we took the nearest integer of each HR and then divided the integers by two. The prognostic score was calculated for each case by summing the partial scores, which ranged from 0 to 7.0. To explore the association between prognostic score and survival time, the survival curves were compared according to different prognostic scores among the study subjects. The survival curves were calculated by the Kaplan–Meier method, and comparisons were based on the log-rank test. The cutoff points for the prediction of survival time shorter than 3 weeks were determined to obtain the highest accuracy for prediction.
All statistical analyses were performed with the SPSS statistical package for Windows version 16.0.2. The significance level was 0.05 for all statistical tests.
Results
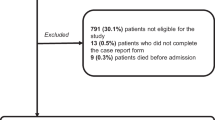
We prospectively followed 209 terminal cancer inpatients in six hospitals from November 2006 to December 2007, until their death in the hospital or the end of this study. Thirty-eight (18.2%) patients were alive at the end of the study or died outside of the hospital; the data from these patients were censored for the purpose of analysis. The median survival time was 26 days [95% confidence interval (CI), 22–29]. The number of men was 100 (47.8%). The median age was 66 years (range, 20–106). The primary site of cancer in descending order of incidence was the lung, hepatobiliary, and stomach (Table 1).
There were no significant differences in survival time according to age, type of primary cancer, or regular opioid use. Table 2 shows the prevalence of variables and the results of univariate analysis of survival time. Reduced oral intake (anorexia) was common and present in up to 48.3% of patients; it was significantly related to a shorter survival (p < 0.001). Highly significant shorter survival times were observed among the following: men (p = 0.006), patients with resting dyspnea (p < 0.001), cognitive impairment (p = 0.001), medium or high levels of pain (NRS, 5–10, p < 0.001), low performance status (ECOG = 4; p < 0.001), increasing number of leukocytes (>11,000/mm3; p < 0.001), elevated serum bilirubin (>2.0 mg/dL; p < 0.001), elevated serum creatinine (≥1.5 mg/dL; p = 0.007), and elevated serum LDH (≥502 IU/L; p = 0.004). Significantly shorter survival times were observed in those patients with treatment other than surgery (p = 0.045), an elevated serum CRP (≥10.3 mg/dL; p = 0.01), and an elevated serum uric acid (≥4.4 mg/dL; p = 0.01).
The results of the multivariate analysis are shown in Table 3. Anorexia (HR = 1.885; p = 0.001), resting dyspnea (HR = 1.676; p = 0.008), low performance status (HR = 2.109; p < 0.001), leukocytosis (HR = 1.733; p = 0.002), elevated serum bilirubin (HR = 1.899; p = 0.004), elevated serum creatinine (HR = 3.872; p < 0.001), and elevated serum LDH (HR = 1.722; p = 0.001) were independent significant prognostic factors of poor survival time.
Based on these results, we established a new, objective scoring system to estimate the survival time of terminally ill cancer patients. In calculating the OPS, we used the seven predictors that were identified as significant indicators of survival time by Cox’s regression analysis. Table 3 shows the partial score for each variable, which was obtained by dividing the nearest integer of each HR by two. The partial score revealed a relationship between the magnitude of the effect on survival time and the prognostic variables.
The new OPS consists of the sum of the anorexia, dyspnea, ECOG, leukocytosis, bilirubin, and LDH scores. The OPS for a given patient was calculated by adding the partial scores, with a range from 0.0 to 7.0. The distribution of the OPS was zero in 13.0%, 1.0∼2.0 in 40.5%, 3.0∼4.0 in 34.6%, and 5.0∼7.0 in 11.9%.
Figure 1 shows the survival curves of two groups with different OPS: the high (OPS ≥ 3.0, n = 82, 46.5%) and low (OPS ≥ 3.0, n = 99, 53.5%) OPS groups. The patients in the former group survived for a significantly shorter time than the latter (p < 0.001), with median survival times ± standard error (95% CI) of 14 ± 1.6 (10.9–17.1) days and 49 ± 8.3 (32.8–65.2) days, respectively. Among the total 209 participants, we excluded cases with missing values for the seven predictors for OPS; 185 participants were included in the final model. On all 185 assessments of the study subjects, the cutoff point to predict whether patients would live longer than 3 weeks was explored. Table 4 shows the sensitivity, specificity, positive predictive value, and negative predictive value with each cutoff point. The cutoff point for OPS was set at 3.0 because this showed the best sensitivity and specificity. The OPS showed acceptable accuracy: The 3-week prediction sensitivity was 74.7%, and the specificity was 76.5% (overall accuracy 75.5%).
Discussion
Survival prediction is difficult even among experienced palliative care doctors [3, 9]. The difficulties include unpredictable causes of death such as embolism or sepsis, individual patient variations, and the complexities of the dynamic dying process itself. Many advancements have been achieved in diagnosis and therapeutics; such advancements should be kept pace with the ability of prognostication [9]. Fortunately many studies on prognostic determinants of survival have been published in the last decade. A recent systematic review recommended the use of a prognostic score with the combination of the CPS as a highly reliable method [18]. Cancer patients have a relatively short terminal phase, and these are the patients often receiving palliative care. Recent review articles have suggested four prognostic scores for all types of cancer in patients receiving palliative care; they include the following [6, 14].
First, the PaP score is the most widely used validated tool; it has been validated in various independent populations [7, 14]. However, the PaP contains the CPS, and the CPS can be difficult for an inexperienced doctor to perform. In addition, the PaP has been associated with a bias toward overestimation of the survival time [15] and subjective disagreement. A prior study found that the CPS was unreliable, especially when patients died within 1 month [10]. The study showed that the CPS was accurate in 55% to 63% of patients and that the survival of patients who lived for no more than 1 month was accurate in only 4.4% to 29% of cases. Moreover, extended clinical experience does not seem to improve the accuracy of the CPS. Considering the limitations of the CPS, the need for a prognostic score not based on the CPS was suggested, especially for those patients near death.
Second, the palliative prognostic index (PPI) does not require the CPS; it is simple to use and has a high accuracy [19]. This index combines the palliative performance score, oral intake, edema, dyspnea, and delirium scales. However, the symptoms can be subjective [14], and in our previous research using the PPI [15], disagreements existed between physicians in the assessment of mild edema. The PPI also has the limitation of being developed by a single study in one hospital.
Third, the cancer prognostic score is a tool that predicts 1- and 2-week survival [5]. This score was developed in a Taiwanese hospital. The inclusion of subjective symptoms, such as feeling tired, reduces the accuracy of the measurements. Another limitation is that this score was developed for patients whose major cause of death was hepatocellular carcinoma. Thus, ascites was included as a predictor of short-term survival. Therefore, the accuracy of this score, for different cancer populations, is questionable.
Fourth, the intra-hospital cancer mortality risk model (ICMRM) has a high predictive accuracy for hospitalized cancer patients [1]. However, the complex formula used to compute survival predictions limits its use. It estimates the probability of short-term survival (median, 8 days). Considering the short median survival, the ICMRM might be a useful tool for patients admitted to the hospital in critical condition.
Although the prognostic scores are known as reliable tools for survival prediction, each tool has its limitations as described above. One of the preceding prognostic scores did not satisfy the necessary sample size requirements. A ratio of the number of events (deaths) to the number of potential predictors is required to be 10 or higher [28]. In addition, most of these instruments were tested in single studies conducted at single sites [14].
Another reason for the need of a new prognostic score is to include novel objective prognostic factors. If a new parameter is more significantly related with survival than existing prognostic indicators, or if it might replace several existing prognostic indicators, then a more accurate and concise prognostic score might be available. The prognostic importance of simple biologic pameters such as LDH and CRP has been reported in many prior studies [2, 6, 13, 25, 26].
Therefore, we developed a new OPS using a prospective multicenter study design. The variables used as predictors were selected from a literature review and discussion based on our clinical and research experience. The predictors that proved significant by the multivariate analysis were anorexia, resting dyspnea, low performance status, leukocytosis, elevated bilirubin, elevated creatinine, and elevated LDH. Anorexia results from the cancer anorexia cachexia syndrome, which is a common cause of death in patients with terminal cancer [27]. Anorexia and resting dyspnea are included in the PaP and PPI also. Our study results were consistent with the results of previous studies [20, 29]. The severity of the dyspnea does not represent pulmonary disease or anemia but rather correlates with the degree of muscle weakness in patients with terminal cancer [22]. Performance status is the most extensively and consistently proven prognostic factor [18]. Leukocytosis is included in the PaP, and its correlation with prognosis has been demonstrated [18]. Hyperbilirubinemia has been shown to be an indicator of advanced malignancy [12] and also has been reported to be a poor prognostic factor [23, 26]. An elevated serum creatinine level might be a sign of multiple organ failure and impending death, it might reflect extensive metastasis. Recent studies have shown that LDH was a useful predictive factor for survival in all types of cancer [10, 25]. LDH is known to reflect the tumor burden and invasive potential of tumors [30]. Thus LDH has been suggested to be a marker of tumor aggressiveness or a sign of multiple organ failure because it is related to the damaged cardiac muscles, lungs, and erythrocytes [1].
The strengths of the OPS are as follows: It is derived from an integrated model using symptoms, performance status, and routine blood tests for inpatients; it can predict the 3-week survival with 76% accuracy with seven predictors. The OPS is a simple-to-use tool, it is available to any doctor, and it is designed to minimize subjective variations. We defined the criteria for the assessment of symptoms and selected variables that were well known to clinicians. The ECOG performance scale is easier to measure than the palliative performance scale or Karnofsky performance status. In order to develop a methodologically sound prognostic score, we calculated an adequate sample size [11] and conducted a prospective multicenter study.
There are several limitations associated with our prognostic score. As a first step for validation, this report introduces the construction of the OPS and tested its validity in a study population (training set). Additional validation on an independent population (testing set) is required and is currently in progress. This study was conducted in one Asian country where cancer prevalence may differ from Western countries. However, the most common cause of death among Korean cancer patients is lung cancer, which is similar to Western countries. The application of OPS to outpatients and nursing home residents requires further research. Although blood tests in terminal cancer patients may raise debate, monitoring laboratory data is an element of routine management of inpatient care. With consideration of the patients’ wishes, available laboratory data could be utilized for accurate prognostication. As a multicenter study, the abnormal criteria for laboratory data could be slightly different from center to center, but the median value of our study data were far beyond the abnormal criteria of all centers. The short-term prediction of 3 weeks might be a limitation, but this may be relevant to those patients with far-advanced cancer. Finally, for patients whose prognostic score was near the cutoff point, the survival time prediction might not be practical.
The OPS might be useful for the education and training of clinicians in palliative care. Clinicians may use the OPS as feedback or a guideline to the CPS. Even though some professionals may oppose accurate medical prognoses and detailed prognostic information, the OPS can assist terminal patients and their families.
Additional research is needed to improve prediction of survival. Comparison among different prognostic scores, investigation into psychosocial factors, and patients with earlier phase advanced cancer require additional research.
References
Bozcuk H, Koyuncu E, Yildiz M, Samur M, Ozdogan M, Artac M, Coban E, Savas B (2004) A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract 58:1014–1019. doi:10.1111/j.1742-1241.2004.00169.x
Brown DJ, Milroy R, Preston T, McMillan DC (2007) The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol 60:705–708. doi:10.1136/jcp. 2005.033217
Christakis NA, Iwashyna TJ (1998) Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med 158:2389–2395. doi:10.1001/archinte.158.21.2389
Christakis NA, Lamont EB (2000) Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ 320:469–472. doi:10.1136/bmj.320.7233.469
Chuang RB, Hu WY, Chiu TY, Chen CY (2004) Prediction of survival in terminal cancer patients in Taiwan: constructing a prognostic scale. J Pain Symptom Manage 28:115–122. doi:10.1016/j.jpainsymman.2003.11.008
Glare P, Sinclair C, Downing M, Stone P, Maltoni M, Vigano A (2008) Predicting survival in patients with advanced disease. Eur J Cancer 44:1146–1156. doi:10.1016/j.ejca.2008.02.030
Glare P, Virik K (2001) Independent prospective validation of the PaP score in terminally ill patients referred to a hospital-based palliative medicine consultation service. J Pain Symptom Manage 22:891–898. doi:10.1016/S0885-3924(01)00341-4
Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, Christakis N (2003) A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 327:195–198. doi:10.1136/bmj.327.7408.195
Glare PA, Sinclair CT (2008) Palliative medicine review: prognostication. J Palliat Med 11:84–103. doi:10.1089/jpm.2008.9992
Gripp S, Moeller S, Bolke E, Schmitt G, Matuschek C, Asgari S, Asgharzadeh F, Roth S, Budach W, Franz M, Willers R (2007) Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 25:3313–3320. doi:10.1200/JCO.2006.10.5411
Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA (1984) Regression modelling strategies for improved prognostic prediction. Stat Med 3:143–152. doi:10.1002/sim.4780030207
Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y (2004) Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol 11:310–315. doi:10.1245/ASO.2004.03.011
Kelly L, White S, Stone PC (2007) The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol 18:1395–1399. doi:10.1093/annonc/mdm138
Lau F, Cloutier-Fisher D, Kuziemsky C, Black F, Downing M, Borycki E, Ho F (2007) A systematic review of prognostic tools for estimating survival time in palliative care. J Palliat Care 23:93–112
Lee IC, Kim CK, Suh SY, Kim YS, Cho KH, Kang HC, Youn BB (2007) Validation of scoring system for survival prediction in terminal cancer patients in Korea. J Korean Acad Fam Med 28:682–689
Llobera J, Esteva M, Rifa J, Benito E, Terrasa J, Rojas C, Pons O, Catalan G, Avella A (2000) Terminal cancer. duration and prediction of survival time. Eur J Cancer 36:2036–2043. doi:10.1016/S0959-8049(00)00291-4
Loprinzi CL, Johnson ME, Steer G (2000) Doc, how much time do I have? J Clin Oncol 18:699–701
Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Vigano A, Larkin P, De Conno F, Hanks G, Kaasa S (2005) Prognostic factors in advanced cancer patients: evidence-based clinical recommendations–a study by the Steering Committee of the European Association for palliative care. J Clin Oncol 23:6240–6248. doi:10.1200/JCO.2005.06.866
Morita T, Tsunoda J, Inoue S, Chihara S (1999) The palliative prognostic index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 7:128–133. doi:10.1007/s005200050242
Palmer JL, Fisch MJ (2005) Association between symptom distress and survival in outpatients seen in a palliative care cancer center. J Pain Symptom Manage 29:565–571. doi:10.1016/j.jpainsymman.2004.11.007
Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, Zaninetta G, Petrella V, Barni S, Zecca E, Scarpi E, Labianca R, Amadori D, Luporini G (1999) A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Ital Multicenter Study Group. Palliat Care J Pain Symptom Manage 17:231–239
Ripamonti C, Bruera E (1997) Dyspnea: pathophysiology and assessment. J Pain Symptom Manage 13:220–232. doi:10.1016/S0885-3924(96)00327-2
Rosenthal MA, Gebski VJ, Kefford RF, Stuart-Harris RC (1993) Prediction of life-expectancy in hospice patients: identification of novel prognostic factors. Palliat Med 7:199–204. doi:10.1177/026921639300700306
Shin HS, Lee HR, Lee DC, Shim JY, Cho KH, Suh SY (2006) Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage 31:493–501. doi:10.1016/j.jpainsymman.2005.11.014
Suh SY, Ahn HY (2007) Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study. Eur J Cancer 43:1051–1059. doi:10.1016/j.ejca.2007.01.031
Suh SY, Ahn HY (2007) A prospective study on C-reactive protein as a prognostic factor for survival time of terminally ill cancer patients. Support Care Cancer 15:613–620. doi:10.1007/s00520-006-0208-5
Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M (2004) Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer 101:1090–1098. doi:10.1002/cncr.20472
Vigano A, Dorgan M, Buckingham J, Bruera E, Suarez-Almazor ME (2000) Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med 14:363–374. doi:10.1191/026921600701536192
Vitetta L, Kenner D, Kissane D, Sali A (2001) Clinical outcomes in terminally ill patients admitted to hospice care: diagnostic and therapeutic interventions. J Palliat Care 17:69–77
Walenta S, Mueller-Klieser WF (2004) Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 14:267–274. doi:10.1016/j.semradonc.2004.04.004
Acknowledgement
This study was supported by a grant from the 2006 Support Fund for Research Group of Korean Academy of Family Physicians. The study sponsors had no role in this study.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suh, SY., Choi, Y.S., Shim, J.Y. et al. Construction of a new, objective prognostic score for terminally ill cancer patients: a multicenter study. Support Care Cancer 18, 151–157 (2010). https://doi.org/10.1007/s00520-009-0639-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0639-x