Abstract
Goals of work
Inoperable bowel obstructions are not uncommon in advanced cancer and are associated with a very poor prognosis. Symptom control includes reducing the frequency of vomiting by prescription of antisecretory medications. The most commonly used agents for this are either hyoscine butylbromide or octreotide. Either histamine 2 antagonists or proton pump inhibitors are sometimes recommended as adjuvants to reduce gastric secretions. The aim of this study was to examine the effects of histamine 2 antagonists and proton pump inhibitors and to objectively compare the effects of one agent over another.
Materials and methods
Previously, electronic databases were searched for trials that compared ranitidine versus proton pump inhibitors in their effect on volume of gastric aspirates.
Results
Seven trials were included in a meta-analysis. Pooled outcomes suggest that both proton pump inhibitors and ranitidine reduce gastric volumes, but the most superior agent is ranitidine, which reduces the volume of gastric secretions by an average of 0.22 ml.kg−1; 95% confidence interval 0.04 to 0.41.
Conclusions
Based on well-conducted studies, objective evidence exists that confirms ranitidine will decrease the volume of gastric aspirates. This forms a sound basis from which to develop further research aimed at improving the care of people with malignant bowel obstructions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inoperable malignant bowel obstructions are likely to occur in up to 50% of people with advanced ovarian cancer and in up to 28% of people with advanced gastrointestinal cancers [1]. Whether operable or inoperable at presentation, malignant bowel obstructions are associated with a poor prognosis, with a mean life expectancy of 3 months or less [2]. The associated symptoms are generally acknowledged as unpleasant and distressing. These may include nausea, vomiting, anorexia, abdominal distension, abdominal pain or discomfort, anxiety and dry mouth [3].
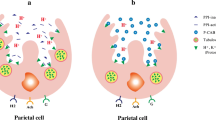
Some supportive and palliative care therapeutic guidelines and formularies advocate the addition of adjuvants such as histamine 2 receptor antagonists (H2 antagonists) or proton pump inhibitors (PPI) to further reduce the volume of gastric secretions [4, 5]. Based on the mechanism of action of these medications, this would seem very reasonable. H2 antagonists block the H2 receptors of the stomach’s parietal cells, thereby inhibiting the stimulatory effects of histamine on the volume of gastric secretions. PPIs block the enzyme system of hydrogen/potassium adenosine triphosphatase (H+/K+ ATPase), the ‘proton pump’ of the gastric parietal cell, such that the stimulatory actions of histamine, gastrin and acetylcholine are all inhibited. Most commonly, these drugs are used to treat peptic ulcer disease, with PPI generally considered superior to H2 antagonists.
However, despite recommendations to add these agents as adjuvants in palliative management of gastrointestinal obstructions, there are no reports of the clinical effectiveness to support this. There is little understanding as to the clinical magnitude of benefit that could be expected or to suggest the superiority of one agent over another.
Previously, we questioned whether H2 blockers or PPI were superior to reduce both the volumes and pH of gastric secretions [6]. The aim of this paper is to consider the data previously collected to examine the effects of H2 blockers and PPIs to reduce the volume of gastric secretions and to compare the effects of one agent over another to identify if one is the superior choice while awaiting adequately powered, definitive phase III studies.
Methods
Search strategy for identification of studies
Previously, Medline, EMBASE and CINHAL were searched for randomised clinical trials that compared H2 antagonists and PPIs given by any route of administration to modify or reduce gastric secretion volumes and pH in humans. The same search was adopted for this paper and used the terms: ‘histamine 2 antagonists’, ‘H2 blockers’, ‘proton pump inhibitors’, ‘gastric secretions’, ‘gastric fluid’ and combinations of these [6].
Eligibility criteria
Only randomised trials that compared any PPI with any H2 receptor antagonist’s effects on gastric volume, measured either in millilitres (ml) or millilitres per kilogram (ml kg−1) were considered. Only published randomised trials in English were retrieved.
Methods of the review
Trial quality was assessed using Jadad scores [7] (Table 1). Other data tabulated included the characteristics and numbers of participants; the duration of fasting and lag time between a medication’s administration and aspiration of the stomach contents; and the name, dose and route of administration of any of the drugs used in the studies. The outcome variable of interest for this paper is the volume of gastric fluid aspirated while the participant had fasted after the medications had been administered.
Statistical analysis
The null hypothesis was that there is no difference between H2 antagonist and PPI to reduce the volume of gastric aspirations. Analysis of data was undertaken using Stata SE 10 statistical software (StataCorp. Stata Statistical Software: Release 10.0: Stata Corporation; 2007. College Station, Texas, USA).
Differences in gastric aspirate volumes after H2 antagonist versus PPI were calculated by comparing the mean difference in gastric aspirated volumes divided by the pooled standard deviation. The ‘standardised mean difference’ (SMD), was used as this allows meta-analysis of trials that used either ml or ml kg−1 as volume measures. Heterogeneity and bias were considered. A graphical and numerical approach was used, with a χ 2 test. The pooled effect allowed the studies to be compared and the total effects calculated with a Z-test. A p < 0.05 was considered significant for all statistical evaluations. Study bias was assessed with funnel plots using the ‘fill and trim’ method, Begg’s rank correlation method to calculate the correlation between the standardised treatment effect and the variance of the treatment effect [8] and finally, the Eggar regression method which fits a linear model of the standardised treatment effect to measure the impact on precision (i.e. the inverse of the variance) [9].
Results
The only studies that could be located that describe the prescription of either H2 antagonists or PPI to reduce volume of gastric secretions were undertaken in the peri-operative period for elective surgery where participants were randomly allocated to receive either a PPI or H2 blocker in the pre-operative phase. The basis of these studies was better to understand risk reduction of aspiration during general anaesthetic. No studies that considered the volume of secretions in the management of impaired gastric emptying in a palliative or other clinical situation were located.
There were 40 articles identified from the search, but only seven were sufficient to meet the pre-determined inclusion criteria of the analysis. This allowed the volume of gastric aspirates of 223 participants who received ranitidine to be compared with 222 participants who received a PPI (omeprazole, lansoprazole, pantoprazole and rabeprazole: Table 1). Although two studies included ranitidine and another H2 antagonist (Table 1), only the data from ranitidine and the PPI were adequate for meta-analysis.
The degree of publication and other potential biases was examined. The funnel plot exhibited a pattern of an inverted funnel, consistent with a lack of bias. This was supported by the Begg rank correlation which yielded a result of an adjusted Kendall’s score of 5 (SD = 6.66) with a Z = 0.75, p = 0.453. The final test of publication bias was the Egger regression plot which yielded a bias coefficient of 1.9; however, it was insignificant. All these results suggested a lack of evidence for any publication bias. There was no significant heterogeneity of the included studies identified (χ 2 = 4.99, p = 0.545) or between the subgroups (χ 2 = 2.47, p = 0.480), allowing pooled analysis to be undertaken.
Clinical differences in the details of the retrieved studies include wide variations in dose of administered medications (Table 1). For ranitidine, the maximum dose used across all studies was comparable to the lowest doses recommended as maintenance therapy for peptic ulcer disease, where for PPIs with the exception of pantoprazole, the doses were up to two to four times higher than the minimum dose for the same indication in one or two divided doses [10]. Pre-operative fasting times varied; two studies failed to record fasting times, and where recorded, fasting ranged from 4 to 10 h. The route of administration of medications differed. In five studies, medications were administered orally and in two, intravenously. The time between administration of medications and aspiration of gastric contents varied from 1 to 6 h.
Meta-analysis of ranitidine versus PPI to reduce gastric volume
The Forest plot (Fig. 1) summarises the results. There was a significant pooled treatment effect (Z = 2.34, p = 0.019) with a standardised mean difference (SMD) of 0.22 (95% confidence interval, CI 0.04–0.41). These results suggested that, on average, the use of PPIs resulted in higher volumes of gastric secretions by 0.22 of a standard deviation unit when compared with ranitidine.
In real terms, the gastric fluid aspirated from the placebo arms of the trials was 0.54 (range 0.00 to 1.07) ml kg−1. The volume of gastric aspirates in those treated with a PPI was 0.410 (range 0.00 to 0.82) ml kg−1, compared to the volume of gastric aspirates of those pre-treated with ranitidine which was 0.16 (range 0.00 to 0.32) ml kg−1.
The meta-analysis demonstrated that the use of ranitidine resulted in reduction of the volumes of gastric aspirate, on average, by an additional 0.22 ml kg−1 in comparison with PPIs.
Discussion
Despite relatively larger equipotent doses of PPIs, ranitidine reduces the volume of gastric secretions to a significantly greater degree based on a meta-analysis of quality phase III studies.
Current practice and the evidence base for this
This empirical evidence for the treatment of malignant bowel obstruction has been identified as lacking in quality [11, 12] but, despite this, has resulted in widely published clinical practise recommendations that are based on either retrospective data or small, uncontrolled prospective studies. The mainstays of non-surgical palliation of malignant bowel obstruction include parenteral analgesia, anti-nauseants and measures aimed at reducing intestinal secretions. Reducing intestinal secretions may help to reduce the frequency of vomiting and may assist in pain control, both the cramping pain of peristalsis and the constant pain and nausea of visceral distension.
Two main classes of medications are considered anti-secretory: the anti-cholinergic medications, particularly hyoscine butylbromide, and the somatostatin analogue, octreotide. Based on the current evidence which is mostly observational, octreotide is probably the superior agent. The clinical reality is the choice of agents remains largely based on local availability, cost and physician preference rather than adequately powered phase III studies [13].
Hyoscine butylbromide is the other most commonly used medication in malignant bowel obstructions when the aim is to reduce gastrointestinal secretions [14]. The use of hyoscine to achieve this contrasts with the results of an earlier study which compared gastric volume aspirates after administration of atropine, hyoscine, glycopyrrolate and placebo in a fasting pre-operative (paediatric) population. There were no statistically significant differences in the volumes of gastric aspirations between the atropine, hyoscine and placebo subgroups [15]. In stark contrast, the glycopyrrolate group achieved a statistically significant reduction in gastric aspirate volumes. Again, to place this in real terms, the mean volume of the control group’s aspirates was 0.60 ± 0.09 ml kg−1, which is similar to the atropine’s 0.42 ± 0.05 ml kg−1 and scopolamine’s 0.45 ±0.05 ml kg−1 groups. The volumes of the glycopyrrolate group were much lower, 0.18 ± 0.05 ml kg−1. It is notable that the reduction in gastric aspirates achieved with glycopyrrolate in this study are very similar to the mean volumes of gastric aspirates achieved with the use of ranitidine (0.16 (range 0.00 to 0.32) ml kg−1) from our review of the data.
Generalizability
The reported studies only evaluated people fasting and subsequently undergoing low-risk anaesthesia. It is not possible from this data to comment on the effect of H2 antagonists or PPIs in a much sicker population, typical of people referred with malignant bowel obstruction for conservative management, given the absence of any studies of these compounds in this population. It is therefore important to consider, in the light of the evidence presented here, the potential role of H2 antagonists and PPIs in the symptomatic management of malignant bowel obstruction given their documented ability to reduce the volume of gastric secretions.
Limitations
All of the studies reported fasting volumes of gastric aspirates, with no indication of how recent oral or parenteral fluids would modify the volumes. The duration of the volume-decreasing effect is not clear from these data. The maximum elapsed time between administration of medication and measurement of gastric aspirates was 6 h. Whilst the volumes at this time were still low, it is not possible to comment on how long this effect would continue to persist. This especially needs objective clarification as H2 antagonists in other situations have been associated with the development of tolerance within hours [16], an effect not reported for PPIs.
Routes of administration in the seven studies varied; however, previous comparisons of oral versus intravenous ranitidine and pantoprazole have not reported any difference in clinical effect [17, 18], with the only reported difference being that the onset of gastric acid suppression is more rapid with intravenous administration. With a bowel obstruction secondary to malignancy, medications would generally be administered parenterally. Ranitidine and PPIs may be administered orally or parenterally without dilution thereby allowing its administration subcutaneously [19].
Implications for practice
Both classes of medications reported in this meta-analysis are relatively well tolerated. The addition or substitution of one of these classes of medications may provide a wider and more cost-effective choice of therapies for this frequently encountered clinical presentation.
Implications for research
It is reasonable to extrapolate from anaesthetic studies in order to generate meaningful clinical studies for people with malignant bowel obstruction. Clinical queries that require answers include time to onset of action and duration of benefit of either H2 antagonists or PPIs in the population with malignant bowel obstruction. Their use in malignant intestinal obstruction needs to be confirmed with studies that have the symptoms that they are most likely to directly address (vomiting frequency and vomiting volume) assessed as the primary outcomes in short- and intermediate-term adequately powered phase III studies.
The observation that glycopyrrolate was associated with significantly reduced volumes of gastric aspirates in a pre-operative fasting (paediatric) population should prompt interest in a comparison with an H2 antagonist. Both ranitidine and glycopyrrolate have quantifiable reductions in gastric aspirate volumes documented, unlike any other medications used to reduce gastric secretion volumes in supportive and palliative care. The fact that both these medications are reasonably inexpensive and have few adverse effects associated with their use should make further study an imperative [20]. Subsequent studies should include octreotide, but because octreotide is so much more expensive, adequate prospective pharmaco-economic assessment is necessary.
In conclusion, this work uses data collected from well-conducted studies to confirm the superior effects of ranitidine over PPI and placebo to reduce gastric secretions. This forms a solid basis to build from to collect further evidence that confirms or refutes the benefits of medications in the palliative care setting. Continually reviewing the scientific basis that underpins recommendations for therapy in supportive care guidelines can improve the quality of care delivered to people with malignant bowel obstruction.
References
Feuer DJ, Broadley KE, Shepherd JH, Barton DP (2000) Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev (4):CD002764
Pameijer CR, Mahvi DM, Stewart JA, Weber SM (2005) Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 35(2):127–133. doi:10.1385/IJGC:35:2:127
Ripamonti C, Bruera E (2002) Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 12(2):135–143. doi:10.1046/j.1525-1438.2002.01103.x
Australian Therapeutic Guidelines Palliative Care 2nd ed. Melbourne, Australia: Therapeutic Guidelines Limited; 2005
Ouellette J, Patterson L, P. T. Fast Fact and Concept #091: Interventional Options for Malignant Upper GI Obstruction. American Academy of Hospice and Palliative Care, (www.epercmcw.edu), 2007.
Clark K, Lam L, Gibson S, Currow D (2009) The effect of ranitidine versus proton pump inhibitors on gastric secretions: a meta-analysis of randomised control trials. Anaesthesia (in press).
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12. doi:10.1016/0197-2456(95) 00134-4
Begg CB (1994) Publication Bias. Russell Sage Foundation, New York City
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Australian Therapeutic Guidelines Gastrointestinal 1st ed. Melbourne, Australia: Therapeutic Guidelines Limited; 2008
Krouse RS (2007) The international conference on malignant bowel obstruction: a meeting of the minds to advance palliative care research. J Pain Symptom Manage 34(1 Suppl):S1–S6. doi:10.1016/j.jpainsymman.2007.04.005
Anthony T, Baron T, Mercadante S, Green S, Chi D, Cunningham J et al (2007) Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage 34(1 Suppl):S49–S59. doi:10.1016/j.jpainsymman.2007.04.011
Ripamonti CI, Easson AM, Gerdes H (2008) Management of malignant bowel obstruction. Eur J Cancer 44(8):1105–1115. doi:10.1016/j.ejca.2008.02.028
Ripamonti C, Twycross R, Baines M, Bozzetti F, Capri S, De Conno F et al (2001) Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 9(4):223–233. doi:10.1007/s005200000198
Salem MR, Wong AY, Mani M, Bennett EJ, Toyama T (1976) Premedicant drugs and gastric juice pH and volume in paediatric patients. Anesthesiology 44(3):216–219. doi:10.1097/00000542-197603000-00010
Hatlebakk JG, Berstad A (1996) Gastro-oesophageal reflux during 3 months of therapy with ranitidine in reflux oesophagitis. Scand J Gastroenterol 31(10):954–958. doi:10.3109/00365529609003113
Pisegna JR (2001) Switching between intravenous and oral pantoprazole. J Clin Gastroenterol 32(1):27–32. doi:10.1097/00004836-200101000-00007
Miller R (1984) Pharmacokinetics and bioavailability of ranitidine in humans. J Pharm Sci 73(10):1376–1379. doi:10.1002/jps.2600731013
Agar M, Webster R, Lacey J, Donovan B, Walker A (2004) The use of subcutaneous omeprazole in the treatment of dyspepsia in palliative care patients. J Pain Symptom Manage 28(6):529–531. doi:10.1016/j.jpainsymman.2004.10.005
Davis MP, Furste A (1999) Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 18(3):153–154. doi:10.1016/S0885-3924(99) 00063-9
Escolano F, Castano J, Lopez R, Bisbe E, Alcon A (1992) Effects of omeprazole, ranitidine, famotidine and placebo on gastric secretion in patients undergoing elective surgery. Br J Anaesth 69(4):404–406. doi:10.1093/bja/69.4.404
Hendolin H, Suojaranta-Ylinen R, Alhava E (1993) Effect of single-dose omeprazole and ranitidine on gastric juice acidity and volume in patients undergoing laparotomy. Acta Anaesthesiol Scand 37(5):484–487. doi:10.1111/j.1399-6576.1993.tb03751.x
Nishina K, Mikawa K, Maekawa N, Takao Y, Shiga M, Obara H (1996) A comparison of lansoprazole, omeprazole, and ranitidine for reducing preoperative gastric secretion in adult patients undergoing elective surgery. Anesth Analg 82(4):832–836. doi:10.1097/00000539-199604000-00027
Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H (2000) A comparison of rabeprazole, lansoprazole, and ranitidine for improving preoperative gastric fluid property in adults undergoing elective surgery. Anesth Analg 90(3):717–721. doi:10.1097/00000539-200003000-00038
Uesugi T, Mikawa K, Nishina K, Morikawa O, Takao Y, Obara H (2002) The efficacy of lafutidine in improving preoperative gastric fluid property: a comparison with ranitidine and rabeprazole. Anesth Analg 95(1):144–147
Memis D, Turan A, Karamanlioglu B, Saral P, Ture M, Pamukcu Z (2003) The effect of intravenous pantoprazole and ranitidine for improving preoperative gastric fluid properties in adults undergoing elective surgery. Anesth Analg 97(5):1360–1363
Goel C, Anand LK, Gombar K (2006) Comparative evaluation of single dose intravenous pantoprazole and ranitidine on gastric pH and volume: a double-blind study. J Anaesthesiol Clin Pharmacol 22(2):145–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, K., Lam, L. & Currow, D. Reducing gastric secretions—a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction?. Support Care Cancer 17, 1463–1468 (2009). https://doi.org/10.1007/s00520-009-0609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0609-3