Abstract
Goals of work
Advanced cancer patients’ perceptions of prognosis, which are often overly optimistic compared to oncologist estimates, influence treatment preferences. The predictors of patients’ perceptions and the effect of oncologist communication on patient understanding are unclear. This study was designed to identify the communication factors that influence patient–oncologist concordance about chance of cure.
Materials and methods
We analyzed audiorecorded encounters between 51 oncologists and 141 advanced cancer patients with good (n = 69) or poor (n = 72) concordance about chance of cure. Encounters were coded for communication factors that might influence oncologist–patient concordance, including oncologist statements of optimism and pessimism.
Main results
Oncologists made more statements of optimism (mean = 3.3 per encounter) than statements of pessimism (mean = 1.2 per encounter). When oncologists made at least one statement of pessimism, patients were more likely to agree with their oncologist’s estimated chance of cure (OR = 2.59, 95%CI = 1.31–5.12). Statements of optimism and uncertainty were not associated with an increased likelihood that patients would agree or disagree with their oncologists about chance of cure.
Conclusions
Communication of pessimistic information to patients with advanced cancer increases the likelihood that patients will report concordant prognostic estimates. Communication of optimistic information does not have any direct effect. The best communication strategy to maximize patient knowledge for informed decision making while remaining sensitive to patients’ emotional needs may be to emphasize optimistic aspects of prognosis while also consciously and clearly communicating pessimistic aspects of prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients’ perceptions of prognosis influence their treatment preferences in advanced cancer [8, 32]. Studies have shown that patients with optimistic perceptions of prognosis are more likely to prefer resuscitation [15], favor life-extending therapy [32], and enter phase I clinical trials [25]. While these treatments offer the hope of benefit, they can have considerable toxicity and impact quality of life. Effective oncologist–patient communication about prognosis is prerequisite to defining goals of care, making treatment decisions, and planning for the future in ways that most accurately reflect patient values and wishes.
Many patients desire that their physicians provide detailed prognostic information in a direct and honest manner [3, 7, 13, 17]. Yet, many do not achieve a clear understanding of their prognosis and commonly overestimate their prognosis when compared to the observed duration of survival [32]. Although oncologists often overestimate the survival time for patients with advanced cancer [5, 11, 24, 27], patients generally report even more optimistic estimates than their physicians [10, 23, 25, 28, 30, 32].
While patient factors such as denial or misunderstanding may contribute to the observed discordance [10], physician communication behaviors such as avoidance of discussing prognosis, withholding prognostic information, or presenting overly optimistic information have also been implicated [1, 9, 20, 31]. Some argue that because patients desire, above all, to maintain hope, oncologists should take liberties with what prognostic information is shared, present information gradually over time, and present information in accordance with patient informational preferences [2, 16, 18]. The best strategies for presenting prognostic information to optimize patient understanding while supporting patient hope are unclear. We studied patient–oncologist pairs with concordant and discordant views of prognosis using a database of audiorecorded encounters to identify the communication factors that may influence concordance about chance of cure.
Materials and methods
This study analyzed encounters recorded during the baseline phase of the Studying Communication in Oncologist Patient Encounters (SCOPE) trial. The complete methods for the SCOPE trial are reported in detail elsewhere [19]. The trial was approved by the Duke University, Durham Veterans Affairs (VA), and the University of Pittsburgh Institutional Review Boards. All participants provided written informed consent.
Study sample and procedures
Oncologists and patients were recruited from Duke University (DUMC), the Durham VA (DVAMC), and the University of Pittsburgh (UPMC). Eligible outpatients were identified by oncologists as patients whom they “would not be surprised if they were admitted to an intensive care unit or died within 1 year.” Patients were required to speak English; receive primary oncology care at DUMC, DVAMC, or UPMC; and have access to a telephone. Exclusion criteria included active psychosis or dementia, hearing impairment, speech disorder, inability to provide informed consent, and patients seen primarily by residents or nonphysician providers.
Demographics were collected from oncologists upon enrollment. Patients were administered surveys assessing demographics and denial before their recorded encounter. Recorded encounters included first time consultations, routine visits, follow-up visits, and visits triggered by change. After each visit, oncologists completed a survey assessing patient diagnosis, extent of disease, treatment intent, and prognosis. This survey included a single response item of chance of cure with 11 response options (0%, 1–10%, 11–20%, etc.) Patient post visit surveys, completed within 10 days after the recorded encounter, included predicted chance of cure using the same 11 response options. Patient and oncologist estimates of chance of cure were made independently, and patients were blinded to the prognostic eligibility criteria.
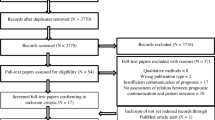
The selection process for the subset of encounters included in this study is shown in Fig. 1. Of the 422 recorded encounters in the SCOPE database, 187 patient–oncologist pairs had estimates of chance of cure reported for both oncologist and patient. Patient and oncologist estimates of chance of cure were compared and pairs were classified as having “good concordance” or “poor concordance” before the analysis of the recorded conversations. Patient–oncologist pairs with responses differing by 0–2 categories (i.e., patient estimated 11–20% chance of cure and MD estimated 0%) were included in the “good concordance” group (n = 69). Pairs with responses differing by 6–10 categories were included in a “poor concordance” group (n = 72). In every “poor concordance” pair, the patient’s estimate was more optimistic than the oncologist’s estimate. The encounters of patient–oncologist pairs with moderate agreement about chance of cure, differing by 3–5 categories (n = 39), were excluded from this analysis to create two distinctly different groups. Six encounters with good (n = 2) or poor (n = 4) concordance were excluded retrospectively from the analysis due to lack of conversation of interest, as discussed below.
The selection process yielded 141 recorded visits between 51 oncologists and 141 advanced cancer patients. The mean number of recorded conversations per oncologist was 2.8 (median = 2) with a minimum of 1 conversation and a maximum of 7 conversations.
Codebook development
We developed a codebook to classify oncologists’ communication behaviors that may affect patient prognostic perceptions. Through the initial analysis of over 50 conversations, we observed that discussions of disease-related prognosis occur rarely; more frequently, oncologist statements concerning the patient’s past, present, and future disease course are made while discussing test results or treatment [29]. We hypothesized that such statements, along with explicit statements about prognosis, influence patient prognostic perceptions. Many of these statements could be classified as optimistic, pessimistic, or uncertain. Based on these observations, a codebook was developed to quantify statements of optimism, pessimism, and uncertainty made by oncologists during discussions of test results, treatment, and prognosis (Table 1). Optimistic statements and pessimistic statements were subclassified as referring to the past or present or referring to the future. Encounters containing no discussion of test results, treatment, or prognosis were excluded from the study (n = 6).
Using this codebook, the content of the recorded conversations was analyzed by two trained coders (T.R. and M.H.) who were blinded to the patient and physician survey responses. All conversations were analyzed by one coder (T.R.) and 15% were coded by M.H. to assess interrater reliability. Disagreements were discussed and final decisions were made by the primary researcher. Cohen’s kappa was used to calculate the interrater reliability for each code using Landis and Koch’s classification (0.21–0.40 = fair agreement, 0.41–0.60 = moderate agreement, 0.61–0.80 = substantial agreement; Table 1) [21].
Measurements and statistical analysis
Analyses were performed using the SAS for Windows Version 9.1 (SAS, Cary, NC, USA). The unit of analysis was the entire recorded encounter. Frequency counts were computed for the number of encounters in which test results, treatment, and prognosis were discussed. The total encounter lengths and percentage of conversation of interest (test result, treatment, and/or prognosis discussion) in each encounter were calculated. Frequency counts of statements of optimism, pessimism, and uncertainty per encounter were compiled.
Predictors of patient–physician concordance about chance of cure
Patient–physician concordance about chance of cure was the outcome variable, analyzed as a dichotomous variable of “good concordance” and “poor concordance” as defined above. The explanatory variables of statements of optimism (total, past/present, and future), pessimism (total, past/present, and future), and uncertainty were initially examined for association with the outcome using chi-square analyses. A large portion of encounters did not have any statements; therefore, the counts of optimism, pessimism, and uncertainty were collapsed to two level variables of “any statements” vs “no statement.” Each explanatory variable demonstrating an association at the p < 0.05 level was entered into a separate multivariable logistic regression model with covariates of interest including patient age, sex, level of education, diagnosis, and denial. The explanatory variable and covariates were entered into models simultaneously. Patient age was analyzed as a two-level variable of <65 and ≥65. Level of education was analyzed as a two-level variable of “high school graduate or less” and “at least some college” because data was sparsely distributed (Table 2). Patient diagnosis was analyzed as a two-level variable of “hematological malignancy” vs “nonhematological malignancy” because treatment of advanced hematological cancers may offer a chance of cure more frequently than treatment of solid malignancies. Patient denial was assessed using the summed score of the two denial items from the brief COPE scale [4]. Patient race was not included in the multivariable logistic regression models as 84% of patients were white.
In addition to the above analysis, we included a random effect for oncologist and fit mixed effects logistic regression models to determine if there was a clustering effect within oncologist; there was no evidence that this effect exists. This suggests that patients consulting the same oncologist were not more likely to have good or poor concordance about chance of cure than patients consulting with different oncologists.
Results
Demographics
The mean oncologist age was 44.9 years (SD = 8.1) and most were men (78%) and white (82%). Specialties included medical oncology (69%), hematological oncology (20%), gynecological oncology (8%), and radiation oncology (4%; Table 2). The average patient age was 60.0 years (SD = 11.2) and the majority were white (84%). Patients had a heterogeneous group of cancers, the majority of which were disseminated at the time of the visit (73%). Twenty-three percent were undergoing treatment with curative intent while the remaining were undergoing palliative (64%) or no treatment (13%; Table 2).
Oncologist communication behaviors
The average encounter length was 19 min and 52 s (SD = 13 min and 51 s). The average percentage of the visit spent discussing test results, treatment, and/or prognosis was 38.0% (SD = 26.3%). Treatment (94.3%) and test results (77.3%) were discussed in the majority of visits, while prognosis was discussed in 50.4% of visits.
Oncologists made optimistic statements about the patient’s cancer in the majority of encounters (85%) with an average of 3.3 optimistic statements made per visit (SD = 3.2). Optimism or reassurance about the past or present course of the cancer was expressed in 58% of encounters with an average of 1.6 statements per visit (SD = 2.1). Optimism about the future was expressed in 60% of encounters with an average of 1.7 statements per visit (SD = 2.3).
Pessimism was expressed less frequently than optimism. Statements of pessimism were made in 46% of encounters with an average of 1.2 pessimistic statements per visit (SD = 2.1). Oncologists very rarely emphasized that the patient’s disease course had not gone well, expressing concern, disappointment, or discouragement about the patient’s cancer in only 15% of visits. Pessimism about the future was expressed more frequently (42% of encounters) than pessimism about the past or present (1.0 vs 0.2 pessimistic statements per encounter).
Oncologists made statements of uncertainty in 36% of encounters with an average of 1.0 statement of uncertainty per visit (SD = 2.3).
Predictors of oncologist and patient concordance about chance of cure
In bivariate analyses, statements of optimism did not increase the likelihood of agreement or disagreement about chance of cure (OR = 0.54, 95%CI = 0.21–1.39; Table 3). In addition, no significant association was demonstrated between concordance about chance of cure and statements of optimism about the past or present (p = 0.700), statements of optimism about the future (p = 0.704), statements of pessimism about the past or present (p = 0.198), or statements of uncertainty (p = 0.988). However, significant associations existed between concordance and statements of pessimism (p = 0.006), and statements of pessimism about the future (p = 0.015).
In multivariable analyses, statements of pessimism and pessimism about the future were significantly associated with an increased likelihood of good concordance about chance of cure independent of patient education, age, gender, diagnosis, or denial (p = 0.006, p = 0.017; Tables 4 and 5). When oncologists made at least one pessimistic statement, the pair was more likely to have good concordance about chance of cure compared to when no statements of pessimism were made (OR = 2.92, 95%CI = 1.35–6.32; Table 4). Similarly, when oncologists made at least one pessimistic statement about the future, the pair was more likely to have good concordance about chance of cure (OR = 2.58 95%CI = 1.18–5.62; Table 5). Patient education, age, gender, diagnosis and denial were not independently associated with concordance about chance of cure.
Discussion
Consistent with previous studies, we found that patients with advanced cancer are often more optimistic about their prognosis than their physicians [10, 23, 28, 32]. When oncologists made at least one statement expressing pessimism, patients were more likely to agree with their oncologists about chance of cure. However, oncologists did not express pessimism in most visits, making pessimistic statements in only 46% of encounters. On average, oncologists expressed more than twice as many optimistic statements as pessimistic statements; however, expressions of optimism did not increase the likelihood that patients would agree or disagree with their oncologists about their chance of cure.
Several previous studies suggest that both communication and patient factors contribute to patient perceptions of prognosis [10, 23, 30]. Our study provides additional evidence to support these findings, specifically in terms of perception of chance of cure, which is most influenced by oncologist statements of pessimism about the patient’s cancer. One explanation for this finding is that oncologists speak more frankly with patients whom they believe have a good understanding of prognosis. However, previous studies have shown that physicians often do not recognize patients’ optimistic misconceptions about prognosis [10, 28], making this explanation less likely.
Alternatively, pessimistic statements may directly inform patients’ perceptions of prognosis. The following statements made while discussing palliative treatment illustrate that oncologists can provide truthful information to patients with or without explicitly stating the pessimistic aspects of the situation.
-
Example 1:
“The (treatment) will control the estrogen receptor positive cancer cells. So that should work nicely.”
-
Example 2:
“One thing that I pointed out before and I just want you to understand that, that although I am trying to give you chemotherapy to control your disease, you are in the stage where I cannot make the cancer go away completely.”
In the first example, the oncologist focused on treatment-related prognosis, expressing the optimistic information that the treatment should control the disease without discussing the disease-related prognostic information that the cancer is likely incurable. Previous studies have shown that this is a common strategy employed by physicians when discussing prognosis with advanced cancer patients [29, 31]. The second example differs in that the oncologist explicitly states the pessimistic aspect of this information; that while the treatment can control the disease, it will not achieve the ultimate goal of curing the disease. Our results suggest that although such statements are not made in the majority of encounters, when made such statements may balance out other factors that lead to overly optimistic prognostic estimates and thereby contribute to a more concordant patient understanding of chance of cure.
Oncologists made statements of optimism twice as often as statements of pessimism in the recorded visits, although they were talking with patients that they would not be surprised if they died within 1 year. Accordingly, previous studies have shown that oncologists have a strong desire to help patients with advanced cancer maintain hope and, therefore, tend to focus on positive aspects of prognosis such as positive treatment outcomes, new therapies, or exceptions to the prognostic statistics [3, 12, 26]. Expressions of optimism were not associated with an increased likelihood of patient optimism about chance of cure, which suggests that it is possible for patients to achieve an understanding of certain aspects of their prognosis (e.g., the cancer is incurable) despite physician expressions of optimism or encouragement about other aspects of the disease or prognosis (e.g., “the current CT scan looks good,” “there are many good treatment options available,” or “treatment may prolong your life”). The majority of patients want their physicians to nurture hope and be optimistic about their likely future [6, 14, 22]. This finding suggests that oncologists can honor this patient preference without affecting a patient’s ability to have a realistic understanding of prognosis.
This study has several limitations. We assessed patient understanding of prognosis using only their estimated chance of cure. Therefore, no specific conclusions can be drawn about the effect of the communication behaviors studied on patient perceptions of other aspects of prognosis, including the chances of treatment prolonging life or the chances of treatment improving the quality of life. Second, we analyzed oncologist–patient communication at a single point in time and, therefore, did not account for previous interactions that may have informed patient perceptions of prognosis. To minimize this limitation, we assessed patient perception of cure shortly after the recorded visit and excluded encounters that did not contain prognostically informative conversation topics. Third, this study did not attempt to quantify the specific types of prognostic information given to patients. Therefore, it was not possible to analyze the effect of the communication behaviors studied independently of the specific prognostic information conveyed. Fourth, we did not look at actual patient outcomes and cannot comment on the accuracy of patient or oncologist prognostic estimates or describe the observed length of survival of enrolled patients. Finally, our study does not assess the relationship between the expression of optimism and pessimism and patient emotional states, which may impact the clinical utility of the results. For example, if expressions of pessimism are associated with increased patient depression or anxiety, an oncologist may or may not want to use such statements as a tool to facilitate patient understanding of prognosis.
When caring for patients with advanced cancer, oncologists have the difficult job of supporting patient hope while also facilitating understanding of prognosis to allow patients to make informed decisions. One strategy used to maintain hope is to emphasize the positive aspects of the disease course and prognosis; and this study provides evidence that oncologists can use this strategy without risking that patients will be overly optimistic as a result. Another strategy used to maintain patient hope is to avoid highlighting or even discussing the negative aspects of prognosis. The findings of this study suggest that this practice may contribute to overly optimistic patient estimates of prognosis. Together, these findings suggest that the best clinical practice when caring for patients with advanced cancer may be to emphasize the optimistic aspects of prognosis, such as the chance of a treatment keeping the disease stable, while also consciously and clearly communicating pessimistic aspects of prognosis, such as the fact that the cancer is incurable.
References
Bradley EH, Hallemeier AG, Fried TR, Johnson-Hurzeler R, Cherlin EJ, Kasl SV et al (2001) Documentation of discussions about prognosis with terminally ill patients. Am J Med 111:218–23
Butow PN, Maclean M, Dunn SM, Tattersall MH, Boyer MJ (1997) The dynamics of change: cancer patients’ preferences for information, involvement and support. Ann Oncol 8:857–863
Butow PN, Dowsett S, Hagerty R, Tattersall MH (2002) Communicating prognosis to patients with metastatic disease: what do they really want to know? Support Care Cancer 10:161–168
Carver CS (1997) You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 4:92–100
Christakis NA, Lamont EB (2000) Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ 320:469–472
Clayton JM, Butow PN, Arnold RM, Tattersall MH (2005) Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers. Cancer 103:1965–1975
Fallowfield LJ, Jenkins VA, Beveridge HA (2002) Truth may hurt but deceit hurts more: communication in palliative care. Palliat Med 16:297–303
Fried TR, Bradley EH, Towle VR, Allore H (2002) Understanding the treatment preferences of seriously ill patients. N Engl J Med 346:1061–1066
Fried TR, Bradley EH, O’Leary J (2003) Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc 51:1398–403
Gattellari M, Butow PN, Tattersall MH, Dunn SM, MacLeod CA (1999) Misunderstanding in cancer patients: why shoot the messenger? Ann Oncol 10:39–46
Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J et al (2003) A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 327:195
Gordon EJ, Daugherty CK (2003) ‘Hitting you over the head’: oncologists’ disclosure of prognosis to advanced cancer patients. Bioethics 17:142–168
Hagerty RG, Butow PN, Ellis PA, Lobb EA, Pendlebury S, Leighl N et al (2004) Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 22:1721–1730
Hagerty RG, Butow PN, Ellis PM, Lobb EA, Pendlebury SC, Leighl N et al (2005) Communicating with realism and hope: incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol 23:1278–1288
Haidet P, Hamel MB, Davis RB, Wenger N, Reding D, Kussin PS et al (1998) Outcomes, preferences for resuscitation, and physician–patient communication among patients with metastatic colorectal cancer. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments. Am J Med 105:222–229
Helft PR (2005) Necessary collusion: prognostic communication with advanced cancer patients. J Clin Oncol 23:3146–3150
Jenkins V, Fallowfield L, Saul J (2001) Information needs of patients with cancer: results from a large study in UK cancer centres. Br J Cancer 84:48–51
Kodish E, Post SG (1995) Oncology and hope. J Clin Oncol 13:1817
Koropchak CM, Pollak KI, Arnold R, Alexander SC, Skinner CS, Olsen M et al (2006) Studying communication in oncologist–patient encounters: the SCOPE Trial. Palliat Med 20:813–819
Lamont EB, Christakis NA (2001) Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med 134:1096–1105
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lobb EA, Kenny DT, Butow PN, Tattersall MH (2001) Women’s preferences for discussion of prognosis in early breast cancer. Health Expect 4:48–57
Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS (1988) Cancer patients’ perceptions of their disease and its treatment. Br J Cancer 58:355–358
Mackillop WJ, Quirt CF (1997) Measuring the accuracy of prognostic judgments in oncology. J Clin Epidemiol 50:21–29
Meropol NJ, Weinfurt KP, Burnett CB, Balshem A, Benson AB 3rd, Castel L (2003) Perceptions of patients and physicians regarding phase I cancer clinical trials: implications for physician–patient communication. J Clin Oncol 21:2589–2596
Miyaji NT (1993) The power of compassion: truth-telling among American doctors in the care of dying patients. Soc Sci Med 36:249–264
Parkes CM (1972) Accuracy of predictions of survival in later stages of cancer. Br Med J 2:29–31
Quirt CF, Mackillop WJ, Ginsburg AD, Sheldon L, Brundage M, Dixon P et al (1997) Do doctors know when their patients don’t? A survey of doctor–patient communication in lung cancer. Lung Cancer 18:1–20
Rodriguez KL, Gambino FJ, Hagerty R, Butow P, Arnold RM (2007) Pushing up daisies: implicit and explicit language in oncologist–patient communication about death. Support Care Cancer 15:153–161
Siminoff LA, Fetting JH, Abeloff MD (1989) Doctor–patient communication about breast cancer adjuvant therapy. J Clin Oncol 7:1192–2000
The AM, Hak T, Koeter G, van Der Wal G (2000) Collusion in doctor–patient communication about imminent death: an ethnographic study. BMJ 321:1376–1381
Weeks JC, Cook EF, O’Day SJ, Peterson LM, Wenger N, Reding D et al (1998) Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 279:1709–1704
Acknowledgements
We are indebted to the patients, oncologists, and clinic staff who graciously allowed us to observe their conversations and in so doing made this study possible. This research was supported by a grant from the National Cancer Institute (R01-CA100387) and a Stead Scholarship from Duke University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, T.M., Alexander, S.C., Hays, M. et al. Patient–oncologist communication in advanced cancer: predictors of patient perception of prognosis. Support Care Cancer 16, 1049–1057 (2008). https://doi.org/10.1007/s00520-007-0372-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0372-2