Abstract
Goal of the work
The quality of life (QoL) of patients with cancer is a major area of concern for both patients and their physicians. The independent contribution of functional impairment and co-morbidity to QoL is unclear.
Materials and methods
We investigated initial global QoL in 477 patients: 195 cancer patients aged 60 years or older (group A), 152 cancer patients below the age of 60 years (group B), admitted as inpatients for chemotherapy initiation and 130 patients aged 60 years or older admitted for non-cancer-related disorders (group C). Global QoL was assessed by the EORTC-QLQ-C30 subscale, functional status by the Karnofsky Performance Scale (KPS) and the Instrumental Activities of Daily Living (IADL) scale, and co-morbidity by the Cumulative Illness Rating Scale (CIRS).
Results
In multivariate analyses, global QoL is significantly associated with KPS, IADL and co-morbidity in group A (r 2 = 0.27), with KPS and IADL in group B (r 2 = 0.23), and with age, KPS and IADL in group C (r 2 = 0.38).
Conclusions
IADL contributes to global QoL in addition to the known effect of KPS. In addition, co-morbidity independently influences global QoL in elderly cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the world’s most common diseases. Cancer incidence rates increase with advancing age [1]. Over 50% of all newly diagnosed cancer patients are aged 65 years or older, with over 60% of all cancer deaths occurring in this population segment. Due to demographic changes, the number of cancer patients will increase within the next few decades, basically affecting elderly people [2].
Quality of life (QoL) is a major area of concern in the treatment of patients with cancer, especially among elderly patients and those treated within a non-curative approach. The concept of QoL supports the patients’ individual perspective on their disease and the way in which it affects them. Only by knowing what factors affect their QoL and how treatment will affect their QoL will they be able to judge the utility of treatment and make a valued decision. Up until now, data collected and published on QoL in patients with cancer have been mainly restricted to adult, middle-aged patients. Little is known about differences between elderly and younger cancer patients in relation to their QoL before initiation of chemotherapy.
Functional status (FS) is a subject of great importance in the care of elderly patients in general, and elderly cancer patients in particular. Impaired FS means a loss of independence. Maintaining or regaining independence has high priority in elderly care. Various scales to assess FS have been established in oncology and geriatric medicine. In oncology, the most widely used scales to document FS are the Karnofsky Performance Status (KPS) [3], Eastern Cooperative Oncology Group (ECOG) Performance Status and the World Health Organisation (WHO) Performance Status. These scales are of prognostic importance for overall survival, especially with regard to early death, and for treatment-related toxicity [4]. Validation for these tests has mainly been carried out on a middle-aged population of cancer patients [5–7]. Other scales to assess FS have been established in geriatric medicine. The most widely used are the Activities of Daily Living (ADL) scale [8] and the Instrumental Activities of Daily Living (IADL) scale [9]. These scales were validated with old and very old people and are a good source of information on the patients’ ability to care for themselves, their health care needs and prognosis of survival. Extermann et al. [10] reported that geriatric (ADL and IADL) and oncological functional assessments (KPS and ECOG-Status) show a significant, but nonetheless limited correlation.
Whereas a number of studies have reported an interaction between KPS, ECOG or WHO-Status and QoL [11], no data have been reported to date on the correlation between geriatric functional scales and QoL in cancer patients, especially when adjusted for FS (as measured by tools established in oncology).
Co-morbidity is defined as the presence of one or more additional diseases in a patient with an index disease. There are different scales available to measure co-morbidity [12]. The number and severity of co-morbidities increase with advancing patient age. Extermann [13] has published an extensive overview of co-morbidity measurements in cancer patients.
One Dutch and one Swedish study have both demonstrated that an increase in chronic health problems is associated with a reduction in QoL, irrespective of age, within the general population [14, 15]. Only very few data analysing association of co-morbidity with QoL have been reported in cancer patients so far. Most describe the effect of co-morbidity on QoL in cancer survivors [16–18]. No data have been published on the effect of co-morbidity on QoL at the time of onset of chemotherapy.
Against this background, we therefore conducted a prospective study assessing FS, with both oncological and geriatric scales, QoL and co-morbidity in an unselected group of cancer patients, independent of gender, age, tumour type, approach to treatment, and stage. A group of elderly patients admitted for non-cancer reasons served as a control.
Materials and methods
The study was conducted in the Department of Haematology and Oncology at the University Hospital Jena, Germany. The study was approved by the Ethics Committee at the Friedrich Schiller University of Jena.
Patients
Patients aged 18+ years, newly admitted to hospital to undergo cancer chemotherapy, were asked to participate in a clinical trial, which included a QoL assessment. Written informed consent was obtained by the study physician after patients had been informed of their cancer diagnosis, with a recommendation to undergo chemotherapy. The study physician (where available) recruited admitted patients consecutively for the trials. Patients were grouped into those aged 60 years and older (group A) and those below the age of 60 (group B). In addition, a group of patients aged 60 years and older, admitted for disorders other than cancer, served as a control group (group C). Age limits were fixed before starting the trial. For all patients, the following data were documented from the patients’ records: gender, age, marital status (married vs not married, living alone, divorced or widowed), diagnosis and—if applicable—tumour type (classified as solid or haematological), cancer stage (UICC for solid tumours and Ann Arbor for malignant lymphoma) and treatment approach (curative vs palliative).
Assessment of functional status
FS was analysed with two different methods: KPS [3] and IADL score [9]. Ratings were carried out before initiation of chemotherapy. IADL activities cover the following areas: using the telephone, shopping for food or clothes, meal preparation, housework, washing clothes, travelling by car or public transport, ability to administer medication and money management. The ability of the patient to perform a particular task was categorised as 0 when he or she was not able to do the task and 1 when able to do so. Values were added to a sum score. For further analysis, patients were grouped into those without limitations in IADL versus those with limitations in IADL who scored less than the maximum sum score of eight points.
Quality-of-life assessment questionnaire
QoL was assessed by means of the EORTC QLQ-C30 questionnaire, version 2 [19]. Calculation of scores was performed in accordance with the EORTC QLQ-C30 scoring manual. The EORTC-QLQ-C30 was conducted before start of chemotherapy in cancer patients, or within a few days after admission in the case of non-cancer patients. The EORTC-QLQ-C30 consists of 15 scales. One scale measures global health status/QoL. This scale is calculated by taking the sum of scores obtained for global health status (Item 29: ‘How would you rate your overall health during the past week?’) and QoL (Item 30: ‘How would you rate your overall quality of life during the past week?’).
Co-morbidity
Co-morbidity was recorded on the Cumulative Illness Rating Scale, geriatric version [20]. Each patient co-morbidity was assigned to one of 14 organ systems and rated from 0 (no co-morbidity) to 4 (extremely severe co-morbidity). In patients with more than one disease in any one organ system, only the most severe one was rated. If any disease could be traced back to the primary disease, it was not recorded as co-morbidity. For further analysis, patients were grouped according to whether or not severe co-morbidity (level 3 or 4) was present.
Statistics
Data management and data analysis were performed with the statistical packages SPSS® Version 12 and SAS® Release 8.02. Fisher’s Exact test for categorical variables and the Wilcoxon Mann–Whitney test for metric variables were used to test statistical significance between groups. For all three patient groups, a correlation matrix using Spearman correlation coefficients was generated for age, IADL, KPS, co-morbidity and global QoL. For the influence of independent variables on global QoL, effect size and p values (Wilcoxon Mann–Whitney test or Kruskal–Wallis test) were calculated (univariate analysis). Variables with a p value < 0.05 were entered in a multivariate linear regression model. Age was also entered in the regression model based on the correlation of the patient’s age and FS.
Results
Patient characteristics
The study included 477 patients: 195 in group A, 152 in group B and 130 in group C. Table 1 summarises patient characteristics. More men were included in the tumour patient group (group A: 58%, group B: 56%), when compared to group C (34%). Differences between younger and elderly cancer patients in terms of FS and co-morbidity were evident, but not between elderly cancer and elderly non-cancer patients. In group C, patient diagnoses were diabetes mellitus (43.8%), heart disease (13.1%), liver/gallbladder/pancreatic disease (12.3%), benign haematological disease (10.0%), gastrointestinal tract disease (7.7%) and other diseases (13.1%).
Global quality of life among patient groups
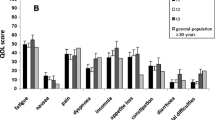
The correlation of the two questions on global health status and QoL was very high for group A (Pearson correlation coefficient r = 0.80) and group C (r = 0.85), but less so for group B (r = 0.69). No difference in global QoL was found between patients in group A (mean = 51.8, SD = 22.2, 95%-CI: 48.7,55.0), group B (mean = 53.0, SD = 23.1, 95%-CI: 49.3,56.7) or group C (mean = 53.5, SD = 22.2, 95%-CI: 49.7,57.4) (Fig. 1). There was no significant difference between younger and elderly cancer patients (p = 0.797) or between elderly cancer and non-cancer patients (p = 0.487). The results are given in Tables 2 and 3, including the univariate analysis of possible factors contributing to global QoL, such as gender, age groups, marital status, diagnosis, tumour type, treatment approach, stage, KPS, IADL and co-morbidity.
Patient group, age and functional status
As FS is known to be a major factor influencing QoL, results for FS and patient groups/age were analysed. The median KPS was 80% (range 20–100%). An increase in age was negatively correlated with KPS/IADL-dependence in elderly cancer (r = −0.264/r = −0.232) and non-cancer patients (r = −0.376/r = −0.340), but not in younger cancer patients (r = −0.049/r = 0.084). Among elderly cancer patients, the median KPS was significantly lower than in younger cancer patients (p = 0.002). No difference in KPS was found between elderly cancer and non-cancer patients (p = 0.733). Only 72% of elderly cancer and 76% of elderly non-cancer patients had a KPS of 80–100% compared to 82% among younger cancer patients. The number of patients classed as being independent on the IADL scale decreased with increasing age; 63% of elderly cancer and 65% of elderly non-cancer patients (p = 0.812), compared with 81% among younger cancer patients (p < 0.001). For elderly and younger cancer patients, correlation of age, KPS, IADL, co-morbidity and QoL are shown in Tables 4 and 5 among elderly non-cancer patients.
Univariate analysis
For all three patient groups, a univariate analysis was carried out, focusing on gender, age, marital status, diagnosis, KPS, IADL and co-morbidity (and if applicable tumour type), treatment approach and tumour stage. In group A, factors found to significantly influence global QoL included KPS, limitations in IADL vs no limitations in IADL, and co-morbidity levels 3–4 vs no co-morbidity levels 3–4. Gender, age, marital status, tumour type, treatment approach, diagnosis and stage of disease had no significant influence on global QoL (see Table 2). In group B, we were able to observe similar results, with the exception that co-morbidity did not contribute to global QoL (see Table 2). In group C, as in the other patient groups, parameters significantly influencing global QoL were KPS and IALD (see Table 3). In all three groups, age as a continuous variable had no effect. In elderly non-cancer patients, the diagnosis was significantly associated with QoL (see Table 3), but not in cancer patients (see Table 2).
Multivariate analysis
All variables showing a significant influence on global QoL in the univariate analysis were included in a multivariate linear regression model (see Table 6). In addition, age was included due to its high correlation with KPS and IADL for elderly cancer and non-cancer patients. For all three groups, KPS and IADL contribute independently to global QoL. In elderly cancer patients, co-morbidity was found to have an additional significant effect. Only in elderly non-cancer patients did age affect global QoL. When controlling for FS, it was found that the older the patients, the better their QoL. The multiple regression coefficient was higher in elderly non-cancer (r 2 = 0.38) than in elderly cancer (r 2 = 0.27) and the lowest in younger cancer patients (r 2 = 0.23).
Discussion
This is one of few reports not to compare healthy individuals with patients, but to investigate differences in global QoL among severely ill patients with or without cancer. Elderly patients in general—and cancer patients in particular—view maintenance or regaining of QoL, as well as independence, as important goals in cancer therapy [21]. Consequently, it is important to know which factors influence QoL in elderly cancer patients. Data obtained on global QoL for the sample of patients investigated were significantly below those for a normal population [22–24]. However, interestingly enough, we could not find any differences between tumour and non-tumour patients, or between elderly and younger tumour patients. Thus, reduction in global QoL seems to be attributed to severe disease requiring hospitalisation, as was the case with our patients, and not to the type of disease. In light of these results, a further control group of younger non-cancer patients would have been interesting. For an outpatient population, Thome et al. [25] reported a poorer QoL in elderly patients with cancer than in a matched group of control patients without cancer.
The influence of FS, measured with tools established in oncology (e.g. KPS or WHO Status), has been described by a number of authors [26–28]. However, Chang et al. did not restrict their analysis to patients recently admitted for treatment. Furthermore, Chang et al.’s data demonstrated that FS correlates with the treatment setting. Only 8% of their outpatients had a KPS below 80%, compared to 41% of inpatients. All of our patients were inpatients and 31% had a KPS below 80%. The influence of FS on QoL, as measured by tools established in geriatric medicine, has yet to be reported. In terminally ill cancer patients, Llobera et al. [29] demonstrated a strong correlation between QoL (as measured by the Hebrew Rehabilitation Center for Aged Quality of life questionnaire or HRCA-QL) and KPS, as well as IADL, but did not report whether such influences are independent of one another. The present study is the first to demonstrate that tools, designed to measure functional reserve and which are established in oncology and geriatric medicine, complement one another, and that one cannot be substituted by the other. In agreement with our data, Hollen et al. [30] could not find any age- or gender-related influence on QoL in their results for patients diagnosed with lung cancer, but did establish such an influence for KPS. Co-morbidity is often present in elderly patients, but its influence on QoL in elderly cancer patients is unknown. As mentioned above, the data reported on co-morbidity and QoL in cancer patients are restricted to cancer survivors [16–18]. Thome et al. [31] reported that co-morbidity in elderly patients with cancer significantly influences QoL, but they did not state at what stage of the disease their analysis was made. Moreover, they did not use a validated co-morbidity scale. Elliot et al. [32] included 405 breast and colon cancer patients with non-metastatic disease within 2 months after diagnosis in their analysis of QoL with reference to other concurrent illnesses. About 50% of their patients were 70 years or older. They considered eight different health conditions and demonstrated that the presence of further health problems negatively influences QoL. However, they did not include a validated co-morbidity scale. Instead of restricting the analysis to a special tumour type or a limited stage of disease, we used a defined time point; i.e. the start of systemic chemotherapy, for the measurement of QoL. Greimel et al. [33] could not demonstrate any interaction between co-morbidity and KPS in their analysis of 227 patients with cancer. In addition, they were unable to demonstrate an age-dependent decline in KPS. This is in contrast to our and other authors’ data. Thus, the elderly patients recruited in their study appear to be highly selected. As demonstrated, IADL and co-morbidities are more sensitive to age-related changes in KPS (see Table 1). Extermann et al. [10] reported that co-morbidity and functional score show only a weak correlation. Therefore, it is advisable to use both a standardised functional assessment and a formal assessment of co-morbidity in elderly patients with cancer [34, 35]. This is supported by our data, demonstrating that co-morbidity influences global QoL in elderly patients with cancer, in addition to KPS and IADL. The fact that co-morbidity independently contributes to QoL in ECP but not in EMP is astonishing and deserves further analysis, e.g. which co-morbidities contribute to QoL.
QoL varies during the course of chemotherapy treatment, due to toxicity and efficacy. Further trials should address the question regarding how geriatric functional scores contribute to QoL during the course of treatment.
In conclusion, this is the first report to
-
(1)
demonstrate that both IADL and KPS independently contribute to global QoL in cancer patients;
-
(2)
show that co-morbidity also contributes to global QoL in elderly cancer patients at the time of commencement of treatment; and is one of few reports that compare global QoL between elderly cancer patients and elderly patients admitted to hospital for disorders other than cancer.
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Edwards BK, Howe HL, Ries LA et al (2002) Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer 94(10):2766–2792
Karnofsky DA, Adelmann WH, Craver FL (1948) The use of nitrogen mustard in the palliative treatment of carcinoma. Cancer 1:634–656
Buccheri G, Ferrigno D, Tamburini M (1996) Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 32A(7):1135–1141
Mor V, Laliberte L, Morris JN et al (1984) The Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer 53(9):2002–2007
Yates JW, Chalmer B, McKegney FP (1980) Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 45(8):2220–2224
Conill C, Verger E, Salamero M (1990) Performance status assessment in cancer patients. Cancer 65(8):1864–1866
Mahoney FI, Barthel DW (1965) Functional evaluation. Md Med J 14:61–65
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(3):179–186
Extermann M, Overcash J, Lyman GH et al (1998) Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16(4):1582–1587
Schaafsma J, Osoba D (1994) The Karnofsky performance status scale re–examined: a cross–validation with the EORTC–C30. Qual Life Res 3(6):413–424
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Extermann M (2000) Measuring comorbidity in older cancer patients. Eur J Cancer 36(4):453–471
Kempen GI, Ormel J, Brilman EI et al (1997) Adaptive responses among Dutch elderly: the impact of eight chronic medical conditions on health-related quality of life. Am J Public Health 87(1):38–44
Michelson H, Bolund C, Brandberg Y (2000) Multiple chronic health problems are negatively associated with health related quality of life (HRQoL) irrespective of age. Qual Life Res 9(10):1093–104
Trentham-Dietz A, Remington PL, Moinpour CM et al (2003) Health-related quality of life in female long-term colorectal cancer survivors. Oncologist 8(4):342–349
Vacek PM, Winstead-Fry P, Secker-Walker RH et al (2003) Factors influencing quality of life in breast cancer survivors. Qual Life Res 12(5):527–537
Sarna L, Padilla G, Holmes C et al (2002) Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol 20(13):2920–2929
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of cancer QLQ–C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16(5):622–626
Pinquart M, Duberstein PR (2004) Information needs and decision-making processes in older cancer patients. Crit Rev Oncol Hematol 51(1):69–80
Hjermstad MJ, Fayers PM, Bjordal K et al (1998) Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ = C30 (+3). J Clin Oncol 16(3):1188–1196
Schwarz R, Hinz A (2001) Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer 37(11):1345–1351
Michelson H, Bolund C, Nilsson B et al (2000) Health–related quality of life measured by the EORTC QLQ-C30—reference values from a large sample of Swedish population. Acta Oncol 39(4):477–484
Thome B, Dykes AK, Hallberg IR (2004) Quality of life in old people with and without cancer. Qual Life Res 13(6):1067–1080
Schag CA, Ganz PA, Wing DS et al (1994) Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res 3(2):127–141
Chang VT, Hwang SS, Feuerman M et al (2000) Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer 88(5):1175–1183
Hollen PJ, Gralla RJ, Kris MG et al (1994) Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the lung cancer symptom scale. Cancer 73(8):2087–2098
Llobera J, Esteva M, Benito E et al (2003) Quality of life for oncology patients during the terminal period. Validation of the HRCA-QL index. Support Care Cancer 11(5):294–303
Hollen PJ, Gralla RJ, Kris MG et al (1999) Normative data and trends in quality of life from the Lung Cancer Symptom Scale (LCSS). Support Care Cancer 7(3):140–148
Thome B, Hallberg IR (2004) Quality of life in older people with cancer—a gender perspective. Eur J Cancer Care (Engl) 13(5):454–463
Elliott BA, Renier CM, Haller IV et al (2004) Health-related quality of life (HRQoL) in patients with cancer and other concurrent illnesses. Qual Life Res 13(2):457–462
Greimel ER, Padilla GV, Grant MM (1997) Physical and psychosocial outcomes in cancer patients: a comparison of different age groups. Br J Cancer 76(2):251–255
Friedrich C, Kolb G, Wedding U et al (2003) Comprehensive geriatric assessment in the elderly cancer patient. Onkologie 26(4):355–360
Extermann M, Aapro M, Bernabei R et al (2005) Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55(3):241–252
Acknowledgement
The study was supported by German Cancer Aid (Grant No. 70-2445-Hö-3). Ulrich Wedding is currently a research fellow of the “Forschungskolleg Geriatrie” of the Robert Bosch Foundation, Stuttgart.
Conflict of interest statement
None of the authors has to indicate a potential source of conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wedding, U., Röhrig, B., Klippstein, A. et al. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients. Support Care Cancer 15, 1097–1104 (2007). https://doi.org/10.1007/s00520-007-0228-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0228-9