Abstract
Goals of work
To evaluate a 12-week home-based walking intervention among breast cancer survivors and to quantify changes in physical activity (PA) behaviors, body weight, and body composition in response to the intervention.
Patients and methods
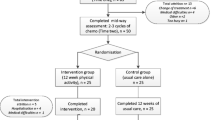
Breast cancer survivors that were in the posttreatment period were randomized to intervention (n=23) or wait-list usual care (n=13). PA was assessed by self-report, and in a study subsample (n=23), by an accelerometer. Intention to treat principles were employed to estimate the intervention effect on PA behaviors, body weight, and body composition. Intervention adherence was calculated as the proportion of exercise sessions completed relative to the number of exercise sessions recommended, as reported each week on walking logs.
Main results
Thirty-four of 36 women randomized (94%) completed the study. Average intervention adherence over 12 weeks was 94%. Intervention participants reported a significantly greater increase in walking for exercise [+11.9 metabolic equivalent (MET)-h/week] over time than did usual care participants (+1.7 MET-h/week, p=0.01). Objective measures of activity also indicated that intervention participants increased their activity levels over time as compared to usual care participants [i.e., counts/min/day and steps/day (p≤0.04)]. No significant changes in body weight or composition were observed.
Conclusion
We found that a 12-week home-based walking intervention was safe and effective for increasing short-term PA levels in breast cancer survivors. Future studies are needed to assess the ability of brief interventions to facilitate the maintenance of increased activity levels and to produce favorable quality of life and risk factor outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer will account for about one third of all cancers diagnosed among women in the United States in 2005, and the majority of these women are expected to have a good prognosis following treatment. Roughly 88% are expected to live at least 5 years, and 77% at least 10 years, after their diagnosis [2]. The current standard of care for nonmetastatic breast cancer involves surgical removal of the tumor, and depending on the characteristics of the tumor (e.g., size, receptor status), surgery may be followed by adjuvant chemotherapy and/or radiation and subsequent hormonal therapy. While these intensive treatments clearly improve long-term survival, the emotional and physical demands of the diagnosis and treatment experience are substantial [25]. For many women, the acute adverse treatment effects resolve in the months after treatment ends, but a number of persistent effects of treatment have been described, including weight gain and changes in body composition (sarcopenia) [6], fatigue [28], and reduced physical quality of life [14].
Because of the potential for physical activity/exercise interventions to address many of these adverse effects of the cancer experience, clinicians and researchers have begun to examine the ability of physical activity promotion programs to improve the emotional and physical quality of life of cancer survivors [7, 8]. Evidence is increasing that physical activity interventions may produce favorable changes in hormonal and growth factor profiles that would be expected to reduce cancer risk [12, 23]. In addition, at least one report has indicated that leisure-time physical activity levels 2 years after diagnosis, at or above currently recommended levels [10 metabolic equivalent (MET)-h/week], were associated with a 30–40% reduction in subsequent breast cancer mortality [15].
Accordingly, simple, effective, and inexpensive physical activity interventions for cancer survivors that can improve quality of life and possibly reduce the risk of early mortality are needed. The purpose of this research was to evaluate the effectiveness of an established home-based walking intervention in breast cancer survivors and to quantify changes in physical activity behaviors targeted by the intervention, as well as changes in body weight and composition that may result from the intervention.
Patients and methods
Study design and objectives
The Breast Cancer Walking Study was a small randomized trial designed to evaluate a 12-week home-based walking intervention among women with early-stage breast cancer. Women were eligible if they had been diagnosed with stage I–III cancer, had completed adjuvant treatment within the last 12 months, were postmenopausal, were free of cardiovascular disease and major orthopedic limitations [4], and were not currently exercising on a regular basis (≥5 days/week).
Participant recruitment
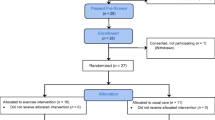
Women were recruited by letter solicitation and phone follow-up in two metropolitan areas, Columbia, SC, and Nashville, TN. SC participants were recruited from the clinical population of the South Carolina Cancer Center with the assistance of their Psychosocial Oncology program. Recruitment efforts in SC were initially designed to enable the evaluation of the effect of the intervention on estrogen metabolism among overweight [Body mass index (BMI)>25] postmenopausal women who did not use medications or dietary supplements that could influence estrogen metabolism. Due to the stringent screening restrictions for this objective, only about 1 in 12 women contacted (8%) were enrolled in the study. To enhance recruitment at the TN site, eligibility criteria related to estrogen metabolism were discontinued, and only criteria related to menopausal status, medical condition, and current exercise status were employed. Women in TN were primarily recruited from a large case control study, 85% of whom consented to be contacted for future research. Participants in this study aged 45 years or older with stage I or II breast cancer were contacted by mail. Of 117 women who were mailed letters, contact was established with 102 of them (87%), and 59 of these women (58%) expressed interest in participating and were screened for eligibility. Twenty three of the women screened were eligible and gave informed consent. Informed consent procedures approved by relevant institutional review boards were completed for each participant.
Randomization and intervention content
Following the collection of baseline measures, participants were randomized (2:1) to a home-based walking intervention (n=22), or to a wait-list control group (n=14). We elected to employ an unbalanced randomization approach to increase the number of eligible participants that received the intervention at baseline. Women randomized to the usual care control condition were asked to maintain their current (baseline) activity levels over the course of the study. Our study team provided no materials or advice about exercise to women in this experimental condition and they were only contacted by study staff to schedule and complete follow-up measurements and appointments at 6 and 12 weeks. However, for ethical reasons, study staff made no efforts after randomization to stop women in this condition from initiating or increasing their activity levels on their own.
Women in the usual care condition received the baseline intervention counseling and materials (e.g., pedometer) upon completion of the study and were offered the opportunity to receive as much telephone counseling as they wanted after this time.
The brief home-based intervention consisted of a single in-person counseling visit (30 min) followed by up to five short telephone-counseling calls in weeks 1, 2, 4, 7, and 10 after randomization (10–15 min/call). The home-based intervention primarily sought to increase walking, and the frequency and content of the behavioral counseling was modeled after the established Stanford University Active Choices program developed by King and colleagues [5]. The initial counseling session emphasized goal setting and physical activity safety (i.e., proper shoes, monitoring exercise intensity, warm-up/cool-down). Subsequent counseling calls were designed to monitor participant safety and enhance adherence through structured behavioral counseling that was grounded in social cognitive theory. A semistructured script was used by the counselors in each of the calls to initiate discussion with participants about their experience in meeting (or not) their walking goals that were agreed upon at the previous intervention contact. Taking their cues from the information provided by the participants in these conversations, the staff then delivered appropriate intervention messages. When participants met their goals, individualized positive reinforcement was provided in the form of a discussion of enjoyment associated with being active and relevant self-rewards. Discussion of personal motivations that helped the individual meet their walking goals was also emphasized. In contrast, if the participant did not meet their walking goals, the conversation naturally led to the barriers participants experienced in the period, and the counselor initiated a conversation about problem solving strategies that might help overcome anticipated barriers in the coming week(s). When appropriate, participants were encouraged to elicit social support from their family and friends that might help them meet their goals (e.g., a walking partner, help with other time commitments). Calls were ended with a recap of the conversation (by the counselor) that included a review of the agreed upon goal for the next week(s), a review of the behavioral issues that were discussed during the call (e.g., positive reinforcements or barriers/problem solving), and an indication of when the next counseling call would occur.
To maximize safety, each call systematically assessed possible cardiovascular and orthopedic symptoms, and participants were instructed to increase their walking frequency, duration, and intensity gradually. When the occasional participant advanced their program too quickly, or reported a high walking volume in a week, the counselor recommended a reduction in their frequency and or daily duration. Pedometers were given as a part of the intervention to assist with the self-monitoring of activity levels. Self-report logs of daily activity/walking, pedometer steps, and ratings of perceived exertion (RPE) during exercise were completed during each week of the intervention. The general walking objectives over the course of the study were as follows: in the first 4 weeks, the goal was to walk three times/week (20–30 min/session); during weeks 5–7, to walk four times/week (30–40 min/session); and for the final 5 weeks of the study, the goal was to walk five times/week (30–40 min/session). Participants were asked to walk at a moderate intensity level as reported by RPE (11–13). Intervention adherence was calculated as the number of completed walking sessions divided by the number of recommended sessions for each week of intervention, as reported on the walking logs. Overall adherence was calculated as the average adherence over the 12-week period. The intervention was delivered by two trained (by S. Wilcox) health counselors.
Demographic and medical characteristics
Demographic characteristics (e.g., age, ethnicity, income, marital status, educational attainment) were evaluated by a self-administered questionnaire. Medical information concerning cancer diagnosis and treatment (i.e., dates, surgery type, treatment regimen) were obtained by a combination of self-report and medical record review. Consent to review medical records was obtained from 31 of 36 (86%) participants.
Physical activity
Physical activity was assessed using both the Community Health Activities Model Program for Seniors Physical Activity Questionnaire (CHAMPS) and, in a subsample (n=23), the Manufacturing Technology (MTI, Fort Walton Beach, FL, USA) Actigraph. Both of these instruments were implemented at baseline, 6 weeks, and 12 weeks. CHAMPS is a comprehensive 41-item self-administered questionnaire that evaluates a wide range of nonoccupational activities, including social activities, recreation and hobbies, housework, walking and jogging, and 14 other types of exercise in the “past 4 weeks.” These data were converted to physical activity energy expenditure (MET-h/week) using MET estimates developed specifically for this instrument [31]. We examined total physical activity and domain specific indices, as well as energy expenditure from walking for exercise derived from three questions related to hiking, walking for pleasure, and walking for exercise.
The Actigraph is a small (2.0×1.6×0.6 in), light (1.5 oz) accelerometer worn on the waist that records minute-by-minute activity information (MTI; model 71256). Participants were instructed to wear the monitor for seven consecutive days on their right hip during waking hours. Monitors were checked for calibration before and after each use. Data were summarized using an automated scoring algorithm that assumes periods of monitor inactivity [0 counts (ct)/m] for 20 consecutive minutes indicate periods of nonwear. Valid days of observation were determined for days on which the monitor was worn for at least 10 h/day (63% of 16 waking hours). The average number of days per assessment period was 6.3 (1.3) days, and the estimated duration of wear was 13.8 (1.3) h/day (values are mean (SD)). Average activity counts (ct/min/day) and duration (min/day) in specific intensity levels [inactive (0–259 ct/min), light (260–759 ct/min), moderate general (760–5,724 ct/min), moderate walking (1,952–5,724 ct/min), and vigorous (+5,725 ct/min)] were calculated for periods when the monitor was worn [21]. Duration data were evaluated in terms of time spent in moderate walking (min/day) and as the proportion of the monitored day spent in specific intensity levels. Steps-per-day values were calculated after censoring cycle count values coinciding with activity counts below 260 ct/min. To objectively estimate walking adherence among women in the intervention, we calculated the frequency (day/week) that women accumulated at least 20 min of moderate-walking time (1,952–5,724 ct/min) using the Actigraph and used this information in comparison to walking objectives at weeks 6 and 12 (e.g., 4 and 5 days/week, respectively).
Body weight and composition
At study baseline, body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.5 cm. BMI was calculated as kg/m2. Body composition was measured for descriptive purposes by two methods. In SC (n=13), bioelectrical impedance (BIA) values were obtained using an RJL Quantum X instrument, and percent body fat was estimated using the equation of Kotler [17]. BIA has been found to be a highly reproducible and reasonably accurate measure of body composition [17]. Data collection in the TN sample was completed using dual X-ray absorptiometry (DEXA) via the LUNAR Prodigy (GE Medical Systems, Madison, WI, USA). Scans were performed in fan beam mode using Lunar software (enCORE 2004, version 8.10.027). The instrument was calibrated daily using the manufacturer’s standard calibration block. Bone mineral density (g/cm2) and content for each calibration scan were consistently within 1% of known values. Bone mineral content was expressed per unit of bone-free fat-free mass (FFM). Determination of fat mass (FM) was based on the extrapolation of fatness from the ratio of soft tissue attenuation of the two X-ray energies in pixels not containing bone. Our laboratory’s intra-assay coefficient of variation for percent body fat using DEXA in adults is 0.79.
Dietary patterns
Changes in major dietary patterns in response to the intervention were evaluated using two brief dietary surveys that were also administered at baseline and at 12 weeks. Women completed the 21-item diet habits questionnaire (DHQ) of Kristal and colleagues [18] and the 19-item National Cancer Institute (NCI) all day fruit and vegetable screener [32]. High scores on the DHQ indicate poor dietary patterns, while higher scores on the NCI screener indicate greater fruit and vegetable intake.
Statistical analysis
Intention-to-treat methodology was used in all primary analyses. Missing observations, including those incurred by participant dropouts, were imputed by the “last observation carried forward” method, and in a single case, a missing baseline Actigraph record (monitor malfunction) was imputed using the participant’s 6-week time point. Baseline values for the demographic, diagnosis, and treatment characteristics and outcome measures were compared between the usual care and the intervention groups using the nonparametric Wilcoxon rank-sum test for continuous variables and chi-square tests or Fisher’s exact tests for categorical variables. To evaluate the effect of the intervention on the outcomes, two repeated-measures analyses were used. For the physical activity outcomes, linear mixed-effect models were used to compare the effect of the intervention over time on the dependent variables (at baseline, 6 weeks, and 12 weeks). The effect of the intervention was assessed by examining the group-by-time interaction, which statistically tests for differences between the regression slopes of the physical activity variables over time between the groups [19]. The covariance structures of the dependent variables were evaluated using Akaike information criteria, and the structure that provided the best fit for the model was employed in the estimation of statistical significance [20]. For the body-composition-dependent variables that were measured at baseline and 12 weeks, we used an analysis of covariance method to test the statistical difference between groups at 12 weeks while controlling for baseline values [13]. Because data for this report were collected at two different sites, using different lead interventionists, and two different body composition measurement methods, we carefully evaluated our results from both sites in separate analyses. There were no major differences in our outcomes between sites; therefore, we chose to combine data from each site in this report. To further minimize the potential for such differences to influence the results, we statistically controlled for study site in all our analyses. Study site was not statistically significant in any of our body composition analyses (all p>0.05) or in our physical activity analyses from CHAMPS, except with regard to household activity (p<0.05). Descriptive analyses of selected characteristics of the intervention as reported on the monthly walking logs were completed using analysis of variance to describe differences between exercise and nonexercise days. Spearman rank correlation coefficients (rho) were employed to evaluate the relationships between self-reported (CHAMPS) and objectively measured activity levels at baseline. A two-sided significance level of 5% was used for all statistical inference. SAS version 8.2 was used for all analyses (SAS Institution, Cary, NC, USA).
Results
At study baseline, there were no significant differences between intervention and usual care groups for any demographic, body size, or medical characteristics examined in Table 1 (all p≥0.13). Only two participants, one in each condition, reported use of aromatase inhibitors at baseline. Women in the intervention received 4.2 (SD=1.3) counseling calls that lasted an average of 15.0 (SD=4.6) minutes each. Total intervention contact time (initial session and calls) was 93.4 (SD=26.6) minutes per participant. Average adherence over 12 weeks to the walking goals of the intervention as reported on the monthly walking logs was 94% (SD=0.48). At study baseline, CHAMPS total activity was positively correlated with Actigraph counts and steps (rho≥0.42, p≤0.05) and inversely associated with time recorded in physical inactivity (rho=−0.49, p=0.02). Self-reported walking on the CHAMPS was strongly correlated with walking as measured by the Actigraph (rho=0.65, p<0.01).
Figure 1 indicates considerable consistency between reported adherence (logs) and that recorded by the Actigraph in weeks 6 and 12 of the intervention, and also highlights a reduction in adherence over time. As the exercise frequency goal increased over time, there was a general downward trend in walking adherence as reported on the walking logs [i.e., 108, 88, and 76% in months 1, 2, and 3, respectively (data not shown)].
Table 2 describes self-reported and objectively measured physical activity over time in the two groups. There were no significant baseline differences in physical activity between intervention and usual care groups (p≥0.20), although women randomized to the intervention tended to report more lawn and garden activity, and, thus, more total activity at baseline. The intervention resulted in a significant increase in self-reported walking in the intervention as compared to the usual care group (group*time p=0.01). Women in the intervention increased the activity behavior targeted by the intervention—walking—from 4.9 MET-h/week at baseline to 16.8 MET-h/week at 12 weeks (Table 2). Overall, reported increases in walking and household activity were responsible for a nonsignificant increase (p=0.10) in total activity on the CHAMPS (+21.5 MET-h/week, Table 2). Figure 2 describes a progressive increase in reported walking among the intervention participants. Total walking time (min/week) reported on the logs also increased significantly in each successive month of the intervention to 103,134, and 147 min/week (p<0.01).
Results from objectively measured physical activity also indicated that women increased their activity levels in response to the intervention (Table 2). Significant group-by-time interactions (p<0.05) were observed for both activity counts and steps. There was also a group-by-time difference of marginal significance (p=0.07) for time recorded in moderate-walking activity. Detailed examination of the Actigraph activity intensity profiles in the two groups, expressed as a percent of total time monitored, suggested that women in the intervention group increased their time spent in moderate–vigorous activity in part by reducing their time spent in physical inactivity (Table 2), while women in the usual care group tended to increase their time spent in inactivity. However, due to the small sample size, these comparisons were not statistically significant.
While there were no significant changes in body weight or composition (Table 3), the usual care group tended to gain fat mass (FM) (0.35 kg) and lose fat-free mass (FFM) (−0.31 kg), but the intervention group tended to lose FM (−0.13 kg) and gain FFM (0.21 kg). In detailed post hoc analyses, we stratified intervention women by reported adherence, and the entire study group by overall activity level, and found no significant or substantive effects of exercise adherence or volume on body composition in either of these analyses (data not shown). We also examined patterns of fruit and vegetable consumption and overall dietary habits in an effort to identify unexpected changes in dietary patterns among participants, and no significant changes were noted (data not shown).
Discussion
The purpose of this investigation was to evaluate the ability of a 12-week home-based physical activity intervention to facilitate the adoption of healthful activity levels in breast cancer survivors and to quantify the intervention effect on physical activity behaviors and body composition. We found that a brief, home-based walking intervention was safe and effective for increasing short-term physical activity levels among breast cancer survivors that were not previously active on a regular basis. In this relatively short intervention that only focused on physical activity, no significant changes in body weight or composition were noted.
We purposely selected a relatively brief, inexpensive, and proven home-based intervention approach [5] that consisted of a single in-person counseling session followed by up to five telephone calls designed to facilitate the adoption of a program of regular walking. Average contact time per participant for the intervention over 12 week was only about 90 min. Jones and Courneya [16] examined the exercise counseling and programming preferences of cancer survivors and found strong preferences (>75%) for the receipt of face-to-face counseling from exercise professionals that were associated with a cancer center, either at the cancer center or at home. They also found clear preferences for walking (81%) and for exercising at home or outdoors (56%) [16]. The intervention approach examined in this research appears to be in close harmony with the preferences of many cancer survivors, and therefore, may be a promising model for delivering physical activity promotion programs for these individuals.
Results from this investigation are largely consistent with other randomized trials conducted among cancer survivors. In terms of adherence to the program objectives, our finding of a 94% adherence rate over 12 weeks was similar to other investigations (75–98%) [10, 11, 26]. In terms of changes in physical activity behaviors, most controlled trials in cancer survivors following adjuvant treatment have achieved exercise levels of 120–160 min/week of moderate exercise by the end of the intervention period [9, 27]. Our finding of an average reported walking time of 147 min/week on the walking logs in the final weeks of the intervention is consistent with these studies. Results from our objective measures of activity were broadly consistent with our self-report instrument, and collectively, our measures indicate that the intervention increased the overall physical activity levels of women in the intervention group.
It is worth noting the probable reasons for the apparent differences between levels of self-reported walking activity over time on the CHAMPS (consistent increase; Fig. 2) and the graded decrease in exercise adherence over time obtained from the walking logs (Fig. 1). We believe that as our goal for exercise frequency increased during the study (from 3 to 5 days/week) there was a general downward trend in walking adherence, as reported on the walking logs (i.e., 108, 88, and 76% in months 1, 2, and 3, respectively). While this trend would appear to be inconsistent with an overall increase in walking (e.g., Fig. 2), the discordance between adherence and our other physical activity outcomes was likely due to the fact that our measure of adherence only considered walking frequency, and not both frequency and duration of activity, as did the CHAMPS instrument. Women increased their overall level of walking each week by increasing walking duration on days that they did walk, but they may not have always met the frequency objectives of the intervention.
The lack of a significant effect on body weight and composition in response to our intervention dose is also consistent with similar short-term, physical-activity-only interventions in cancer survivors in some [9, 11], but not all, studies [29]. McTiernan and colleagues [22] evaluated a combined diet and exercise intervention in breast cancer survivors and found the intervention to result in a loss of about 1.2 kg of body mass over 8 weeks. Longer-term studies, the addition of calorie restriction to the intervention, or a larger volume of exercise, is probably needed to achieve significant weight loss, particularly in short-duration interventions such as this one [1].
Our intervention focused on increasing the activity levels of breast cancer survivors that were not regularly active to levels currently recommended for health promotion and disease prevention [3]. The intervention resulted in an average increase in walking by about 12 MET-h/week and 76% of women in the intervention reported walking on 5 days/week or more in the last 4 weeks of the intervention. Detailed analyses of the intervention participants’ walking logs suggested that they walked at a moderate intensity (RPE 12–13), and on days in which they walked for exercise, they reported pedometer readings that were about 3,500 steps greater than those on their nonexercise days.
The strengths and limitations of this report should be considered carefully. The strengths of this research include its randomized design among physically inactive women and an analysis plan that adhered to intention-to-treat principles. Our finding of a significant increase in physical activity in the setting of a randomized trial supports the idea that physical activity promotion programs in similar populations of cancer survivors may be effective in facilitating the adoption of healthy levels of activity. In addition, we completed comprehensive measurements of physical activity patterns using established instruments, and both our objective and self-report measures of walking were sensitive to changes in activity. This finding is also important because it suggests that a less costly measure of physical activity (CHAMPS) is valid and sensitive to change in physical activity interventions with breast cancer survivors. There are also a number of limitations of this research that should be considered. First, to ensure participant safety in this pilot study, women with existing cardiovascular disease and orthopedic limitations were excluded from participation. In addition, we included primarily women with early-stage breast cancer; about a third of whom received chemotherapy. It is possible that women with more advanced disease that receive more intensive treatment regimens and that have more comorbidities will have lower adherence and possibly higher levels of adverse events. Thus, our results are most generalizable to healthier breast cancer survivors with few significant comorbid conditions. Second, many of our participants enrolled at the TN site were contacted using a list of participants from a breast cancer case-control study. Therefore, it is possible that these women may have been particularly motivated to adopt the walking program. However, our participation rate among women screened in TN [23 of 102 (22.5%)] was only marginally higher than that of other trials among postmenopausal breast cancer survivors (14.3–20.3%) [11, 27]. This suggests that the selection of more motivated women in our study is similar to that found in other published reports. A third weakness of this report is the length of the intervention and follow-up period (12 weeks). The short duration of the follow-up period limits our understanding of the ability of this brief intervention to positively influence the long-term maintenance of healthy activity patterns, or to evaluate the impact of these behavioral changes on long-term energy balance and other outcomes relevant to cancer survivors. Finally, like many other studies among cancer survivors, our sample size in this study was relatively small. While we were still able to measure statistically significant changes in behaviors targeted by the intervention, intervening on a larger, more heterogeneous group of breast cancer survivors may have produced less of an intervention effect than that which was obtained in this study.
Studies examining exercise interventions during and after active adjuvant treatment have reported a number of beneficial effects on physical health and quality of life, including increased cardiorespiratory fitness [11, 24, 26] and a range of psychological and quality-of-life outcomes, including enhanced body image [26], reduced depression and anxiety [30], and better overall quality of life and physical well-being [11]. Collectively, these studies support the concept that physical activity/exercise interventions can produce favorable changes in both the physical and emotional quality of life. Additional research is also needed to test the hypothesis that long-term changes in physical activity behaviors in response to a brief home-based intervention can improve the physical and emotional quality of life of survivors, as well as their risk for recurrence as indicated by relevant surrogate end-point biomarkers (e.g., growth factor profiles, breast density), and trials are underway to answer some of these questions. Future reports from the Breast Cancer Walking Study will describe the effects of the walking intervention on quality-of-life outcomes, as well as predictors of intervention adherence. We are particularly interested in understanding more completely the important moderators and mediators of this intervention so that we can tailor our counseling approach to more effectively meet the needs of subgroups within the population, and also to describe the psychological constructs through which the intervention works.
In summary, we found that a brief, home-based walking intervention was effective for increasing short-term physical activity levels among previously inactive early-stage breast cancer survivors. Intervention adherence rates were similar to those reported in other studies among adults with and without cancer. However, no significant changes in body weight or composition were noted over this short intervention period.
References
ACSM (2004) ACSM position stand on the appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports 33:2145–2156
American Cancer Society (2005) Cancer facts and figures—2005. American Cancer Society, Atlanta, GA
Byers T, Nestle M, McTiernan A, Doyle C, Currie-Williams A, Gansler T, Thun M (2002) American cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk for cancer with healthy food choices and physical activity. CA Cancer J Clin 52:92–119
Cardinal BJ, Esters J, Cardinal MK (1996) Evaluation of the revised physical activity readiness questionnaire in older adults. Med Sci Sports Exerc 28:468–472
Castro CM, King AC (2002) Telephone-assisted counseling for physical activity. Exerc Sport Sci Rev 30:64–68
Chlebowski RT, Aiello E, McTiernan A (2002) Weight loss in breast cancer patient management. J Clin Oncol 20:1128–1143
Conn VS, Hafdhal A, Porock DC, McDaniel R, Nielsen PJ (2006) A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer 14:699–712
Courneya KS (2003) Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc 35:1846–1852
Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS (2003) A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care 12:347–357
Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Handman M (2003) The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: physical fitness and quality of life outcomes. Psycho-Oncology 12:357–374
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS (2003) Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol 21:1660–1668
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR (2003) Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomark Prev 12:721–727
Frison L, Pocock SJ (2005) Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 11:1685–1704
Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR (2004) Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst 96:376–387
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293:2479–2486
Jones LW, Courneya KS (2002) Exercise counseling and programming preferences of cancer survivors. Cancer Pract 10:208–215
Kotler DP, Burastero S, Wang J, Pierson RN Jr (1996) Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr 64:489S–497S
Kristal AR, Curry SJ, Shattuck AL, Feng Z, Li S (2000) A randomized trial of a tailored, self-help dietary intervention: the puget sound eating patterns study. Prev Med 31:380–389
Laird NM, Ware JH (1982) Random-effects models for longitudinal data. Biometrics 38:963–974
Littell R, Milliken G, Stroup W, Wolfinger R (1996) SAS system for mixed models. SAS, Cary, NC, p 633
Matthews CE, Ainsworth BE, Hanby CL, Pate RR, Addy C, Freedson PS, Jones DA, Macera CA (2005) Development and testing of a short physical activity recall questionnaire. Med Sci Sports Exerc 37:986–994
McTiernan A, Ulrich C, Kumai C, Bean D, Schwartz R, Mahloch J, Hastings R, Gralow J, Potter JD (1998) Anthropometric and hormone effects of an eight-week exercise-diet intervention in breast cancer patients: results of a pilot study. Cancer Epidemiol Biomark Prev 7:477–481
McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, Potter JD, Schwartz RS (2004) Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res 64:2923–2928
Mock V, Pickett M, Ropka ME, Muscari LE, Stewart KJ, Rhodes VA, McDaniel R, Grimm PM, Krumm S, McCorkle R (2001) Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract 9:119–127
Partridge AH, Burstein HJ, Winer EP (2001) Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monographs 30:135–142
Pinto BM, Clark MM, Maruyama NC, Feder SI (2003) Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-Oncology 12:118–126
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH (2005) Home-based physical activity intervention for breast cancer patients. J Clin Oncol 23:3577–3587
Schwartz AL (2004) Physical activity after a cancer diagnosis: psychosocial outcomes. Cancer Investig 22:82–92
Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R (2001) Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol 19:657–665
Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, Haslanger S, Wilkins EG (1998) The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum 25:107–113
Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL (2001) CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 33:1126–1141
Thompson FE, Subar AF, Smith AF, Midthune D, Radimer KL, Kahle LL, Kipnis V (2002) Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J Am Diet Assoc 102:1764–1772
Acknowledgements
We would like to thank Ms. Amy Skiba, M.S., for her dedicated efforts on behalf or the Breast Cancer Walking Study. This research was supported financially by the Vanderbilt-Ingram Cancer Center, the South Carolina Cancer Center, and the Vanderbilt General Clinical Research Center (M01 RR-00095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthews, C.E., Wilcox, S., Hanby, C.L. et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer 15, 203–211 (2007). https://doi.org/10.1007/s00520-006-0122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-006-0122-x