Abstract
Goals of work
The goal of this study was to explore living conditions among disease-free cancer survivors participating in the labour force after successful primary treatment. Their living conditions were compared with the conditions of matched controls from the general Norwegian population.
Patients and methods
Living conditions are social indicators that stimulate social inclusion and reduce exclusion such as economy, employment, health, housing and social participation. A questionnaire covering living conditions with established questions from population surveys was mailed to 852 cancer survivors and 1,548 controls. Valid responses were obtained from 51% cancer survivors (216 women with breast cancer, 49 men with prostate cancer, and 165 with testicular cancer) and 39% controls (317 women and 279 men).
Main results
Compared to their controls cancer survivors showed no difference in work hours or full-time jobs, but reported significantly poorer physical and mental work capacity. This was associated with significantly more somatic diseases among survivors and poorer general health status in male survivors. The survivors had significantly smaller households and more living space than controls. No significant differences were observed concerning economy or social participation, except that significantly more female survivors than controls stated that they had enough friends.
Conclusions
In spite of poorer health, tumour-free survivors after breast, testicular, and prostate cancer report mostly equal living conditions compared to matched controls. The protection hypothesis of holding jobs as a precondition for normal living condition was confirmed in our study of a sample of cancer survivors with good outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, the concept of an individual’s welfare consists of living conditions and quality of life, but until now, only the latter aspect has gotten any considerable attention in cancer research. In the context of sociological research, living conditions include the social indicators that stimulate social inclusion and reduce social exclusion. These indicators are economy, education, employment, health, housing, and social participation [2]. The comparison of living conditions between countries faces particular difficulties due to considerable differences in the organization of health care systems, benefits and insurances, climate, culture, and social networks.
As a result of early diagnosis and effective multimodal treatment about 60% of today’s cancer patients in the Western world are alive 5 years after diagnosis, although not all of them are without evidence of disease. Being diagnosed with cancer nowadays is frequently like facing a chronic disease [4].
Among those diagnosed with cancer in Norway between 1998 and 2002, 15% of the men and 24% of the women were younger than 55 years. These proportions indicate that a considerable number of newly diagnosed cancer patients are members of the labour force, in particular females with breast or gynecological cancer. Furthermore, the number of young cancer survivors is growing because of reduced mortality rates in various forms of childhood and adolescent cancer [8].
Due to this development with improved treatment and increased survival rates, more research has been performed during recent years on selected aspects of the living conditions of cancer patients, especially work and economy [1, 5–7, 13, 15, 16, 19, 23, 25]. Concerning cancer and work life Steiner et al. [23] have identified 18 studies published between 1966 and 2003 that primarily focus on the work outcome of cancer survivors, and they concluded that most of them had considerable methodological limitations. A recently published Finnish registry study of cancer survivors in general [24] and a comparative study from the USA of breast cancer survivors [5] reported that the probability of being at work was about 9–10% less after cancer treatment compared to matched controls.
Other studies on cancer survivors have dealt with the relation between health and work, housing, and social participation after primary cancer treatment [4–7, 15, 16, 19, 23, 25]. Case stories and analytic studies have shown that, although still belonging to the labour force, cancer survivors may face poor economy and decreased living conditions as a result of reduced income due to periods of sick leave or rehabilitation, and they also may have increased expenses for health care [14, 17, 23].
So far, there has been a lack of quantitative research on the living conditions of Norwegian cancer survivors. Therefore, the aim of the present study was to explore living conditions among Norwegian disease-free cancer survivors participating in the labour force after successful primary treatment and to compare the findings to a gender-, age-, and municipality-matched control sample from the general population also participating in the labour force. We held the hypothesis that there would be no significant differences between cancer survivors and controls in these regards because we believed that holding a job protected cancer survivors against reduced living conditions (‘the protection hypothesis’).
Methods
The Nordic Study
This study is a part of The Collaborative Nordic Study of Cancer and Work Life, which examines the work situation of cancer survivors in Denmark, Finland, Iceland, and Norway, to explore the problems that cancer patients face in returning to work and coping with working life. The background of the Nordic study was the assumption that cancer patients had markedly reduced opportunities in the labour market, although they were disease-free after successful primary treatment. Each country had the opportunity to perform subinvestigations of national interest, and this study of living conditions was such a project.
Eligibility criteria
The eligibility criteria of the Nordic cancer study were: (1) A primary cancer diagnosis at an age between 25 and 57 years at the time of diagnosis; (2) Primary treatment started between 1998 and 2002 almost 2–6 years prior to the conduction of the survey; (3) No evidence of any invasive malignant disease after the primary treatment except basocellular cancer of the skin; and (4) Ongoing adjuvant systemic hormone treatment was allowed for, but all adjuvant chemotherapy should have been terminated.
The survivors of the present Norwegian study were sampled from the patient registry of Rikshospitalet-Radiumhospitalet Trust and consisted of women with breast cancer stages I and II, and men with prostate cancer or testicular cancer, considered as survivors mainly with good prognosis.
Statistics Norway, which is the national organization for official statistics, identified controls from the general population matched with the cancer patients on age, gender, and municipality of living.
Questionnaires
The questionnaire of the Nordic Study used a series of items from validated questionnaires covering socio-demographic variables, employment, work situation and career, health and well-being, and benefits from the national health insurance. The Norwegian subinvestigation included a special questionnaire with items on living conditions used in the regular population surveys performed by Statistics Norway and the National Institute for Consumer Research.
Variables
Demography and housing
The following variables from the questionnaires were used: age at survey, education (dichotomized into 9–13 years vs >13 years), civil status (dichotomized into paired and non-paired), and status of housing (dichotomized into owned or rented living place). The number and type of the household’s members (number of children ≤17 years old vs adults ≥18 years old who lived in the household) were registered. The size of the house/apartment in square metres was asked for and used to calculate the mean space in square metres per family member as an expression of living in close quarters.
Work and economy
An individual’s work involvement was assessed as the hours of paid work per week [dichotomized into ‘full-time’ (≥37.5 h/week) or ‘part-time’ work (<37.5 h/week; pensioners are allowed to work part-time in Norway)]. Physical and mental work capacity was self-rated on Likert scales with five response alternatives from very good to poor.
The self-reported annual household income was dichotomized above or below the median income of the total sample (EUR 62,500). The respondents also reported on their national insurance benefits (sick leave, rehabilitation support, unemployment sustenance, pensions, and other benefits). In addition, they rated eventual financial worries during last year (grouped as never, sometimes, or often) and their housing expenditure per month [grouped as low (<EUR 600), medium (EUR 600–1,500), or high (>EUR 1,500)].
The socio-economic status was divided into three social classes based on self-rated information about the profession of the individual following the recommendation promoted in a World Health Organization (WHO) report [20] and constructed as an approximation to the international Erikson Goldthorpe Portocare social class schema [10, 11, 21, 22] by using the Occupation Classification 2000 [23]. The sample was grouped as follows: Social class I [higher-grade professionals, directors, and higher legislators, seniors officials, managers, self-employed higher-grade professionals, and liberal professionals (e.g. physicians, teachers, and lawyers); technicians, associate professionals, e.g. opticians, nurses, and superiors at bank and post offices]; Social class II [clerks (e.g. bank clerks and secretaries), service and care workers, shop and market sales worker (e.g. cooks, waiters, hairdressers, police officers, and salespersons), and armed forces]; and social class III: workers [other self-employed, skilled agricultural and fishery workers, craft and related trade workers (e.g. bricklayers, painters, and machinery mechanics), plant and machine operators and assemblers, elementary occupations (e.g. cleaners, kitchen helpers, and messengers)].
Health
The questionnaire also contained ten items about various common symptoms of long standing such as tiredness, nervousness, problem concentrating and keeping thoughts together, headache, palpitations, vertigo, nausea, chest pain, stomachache, and insomnia. The symptoms had five response alternatives from 0 (never) to 4 (all of the time). The overall somatic symptom level was defined as the sum score of these ten item scores [from 0 (no symptoms) to 40 (maximum symptoms)]. Subjective health status was self-rated on Likert scales with five response alternatives from very good (1) to very bad (5) but was recoded into three categories: very good/good (1), moderate (2), and very bad/bad (3).
Concerning diseases diagnosed or treated by a physician, the respondents reported on injuries, musculoskeletal disease, cardiovascular disease, respiratory disease, mental disorder or severe mental health problems, metabolic disease, neurological or sensory disease, or other severe diseases. The total number of diseases reported was dichotomized into none or at least one disease(s) for each respondent.
Social participation
Social relations and activities were rated according to the number of close friends one could talk personally with. Social participation was rated into three categories: at least once a week, one to two times a month, or never/very seldom based on contact with family, neighbors, friends, membership of clubs, voluntary societies, participation in the activities of churches, trade unions, and political parties [1]. The respondents also rated whether social visits were reduced or not due to poor health.
Procedure
The questionnaire was mailed to 852 cancer survivors, 427 women with locoregionally confirmed breast cancer and 425 men with non-metastatic prostate cancer (N=110) or with testicular cancer (N=315) (Fig. 1). Non-responders received one reminder after 4 weeks. All patients with breast cancer had local surgery (either mammary ablation or breast-conserving surgery) with or without axillary lymph node dissection according to standard therapeutic guidelines. Depending on stage, histological grade, and/or hormone receptor status of the tumor tissue, adjuvant chemotherapy and hormone treatment was applied. Patients with prostate cancer had either retropubic radical prostatectomy or pelvic high-dose radiotherapy, which in high-risk patients was combined with adjuvant androgen deprivation for 3 years. After orchidectomy patients with testicular cancer either entered a surveillance programme, had infradiaphragmatic radiotherapy, or chemotherapy followed by resection of residual tumors masses, dependent on stage and histology.
We dichotomized the follow-up time interval from the start of the primary treatment to the survey at the median of 4.0 years into short and long follow-up, and tested this variable on the results in male and female survivors.
The same questionnaire without cancer-related items was sent by Statistics Norway to 1,548 (777 women and 771 men) age-, gender-, and municipality-matched controls who were asked to fill in and return anonymously the completed questionnaire in a prepaid envelope.
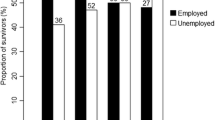
A valid questionnaire contained data on all but one of the variables used in this study. Among the 852 cancer survivors addressed, a total of 513 (60%) survivors delivered a valid questionnaire, and 339 (40%) declined, did not respond, or delivered an invalid questionnaire. Among the 1,548 controls, 700 returned a valid questionnaire (45% response rate) and 848 did not (Fig. 1).
When the 513 complying cancer patients were compared to the 339 non-compliers, no significant differences were observed as to the variables that could be checked in both groups, namely, age, gender, cancer type distributions, cancer stage at diagnosis, and stage at primary treatment. This study includes only those 430 (84%) of the 513 cancer survivors and 596 (85%) of the 700 controls who delivered valid questionnaires on living conditions and were in paid work at the time of the survey.
Statistics
The completed questionnaires were scanned into an electronic database for statistical analyses. The calculations were performed on SPSS for PC version 12.0. Continuous variables were analysed by t tests, and categorical variables were analysed by χ 2 tests. Since the female survivors were significantly older than their controls, statistical analyses with significant results were age-adjusted for the female groups. The findings in survivors were also tested for the influence of time since primary treatment. The significance level was set at p<.05, and all tests were two-tailed.
Ethics
The study was approved by the Regional Committee for Medical Research Ethics of South Norway and approved by the Norwegian Data Inspectorate (NDI). All patients consented to participate in the study by completion of a consent form. NDI did not allow sending a reminder to the controls.
Results
Socio-demographic and cancer data
The 216 working female cancer survivors had a significantly higher mean age than their controls. A significantly higher proportion of male survivors did not live in paired relations (Table 1). No significant differences were observed between the survivors and their controls as to their level of education, civil status, or number of children staying at home, although the male survivors had more children at most 18 years at home than the female survivors (p≤.001).
The mean time from primary treatment to the survey was 4.1 (SD 1.2) years for men and 3.9 (1.4) years for women (p=0.08). An association for follow-up time in living conditions was only found in at least one disease in female survivors and showed that those with short interval had more diseases [67 (60%) vs 45 (44%), p=.02)]. Female survivors had poorer mean scores for physical work capacity due to cancer than males both at short-term [2.0 (SD 1.0) vs 1.7 (SD 1.0), p=.02] and long-term [2.1 (SD 1.1) vs 1.7 (SD 0.9), p=.004] follow-up.
Housing conditions
No significant differences were observed between the survivors and their controls concerning living in owned or rented living places (Table 1). However, the households of the cancer survivors of both genders were significantly smaller than among controls, and the persons living in the cancer survivors’ households had significantly more space per household member. However, these significant findings did not hold up for women after age adjustment.
Work conditions
Cancer survivors and controls did not show any significant differences to the number of paid work hours per week or the proportions of those holding full- or part-time jobs for either gender (Table 2). Female survivors belonged to significantly lower social class, and they reported lower working capacity (both physical and mental) than their controls (also when controlled for age).
Economy
The grouped annual household income levels showed no significant differences. We did not find any significant difference between the cancer survivors and their controls concerning national insurance benefits, financial worries, or housing expenditures (Table 3).
Health status
Cancer survivors of both sexes had significantly more co-morbid diseases (also when age-adjusted in women). The male survivors had significantly worse subjective health status and higher somatic symptom levels than their controls (Table 4).
Social participation
Significantly more female cancer survivors reported that they had enough friends compared to the controls, and age adjustment did not change this finding. On other variables (number of close friends, social participation, and social visits), no significant differences were observed between the cancer survivors and the controls in any of the sexes (Table 5).
Discussion
In this controlled study of living conditions in cancer survivors participating in the labour force, there were no differences in socio-demographic characteristics between the survivors and their controls except that the female controls were significantly younger than the survivors. Our main finding was that cancer survivors, 2–6 years after their treatment, worked closely the same mean number of hours per week, although they reported poorer mental and physical work capacity and more diseases. The cancer survivors’ economic level was similar to that of the controls. Survivors lived with significantly bigger households and space per household member than their controls without significant differences in housing expenditures. Social participation did not differ between survivors and controls for either gender, with the exception of female survivors who more frequently reported to have enough friends compared to controls. Time from start of primary treatment hardly influenced living condition to any significant extent. In general survivors seem to have equal or similar living condition as their controls when they stayed within the work force.
The proportion of cancer survivors active at work is almost the same as that for the matched controls without cancer (85 and 84%, respectively). According to information for Statistics Norway in 2004, this number in the general population is 82% in the age group 25–64 [22]. Our finding that most recovered tumor-free cancer survivors return to work after treatment is in accordance with other studies [4, 7, 15, 19]. In 2004, Maunsell et al. [17] reported from Canada that the working conditions of breast cancer survivors 3 years after diagnosis were mostly unchanged, and no statistically significant difference was observed in the overall working conditions compared to women in the control group.
Work is a major way to get the resources for participation in many levels of society. Our study demonstrates that work participation in cancer survivors is associated with significantly poorer mental and physical work capacity compared to controls. This result raises the question on whether the survivors will be able to keep their jobs until the ordinary retirement age of 67 years in Norway.
Housing is an important domain of living conditions, particularly in a country like Norway, where cold winters make heavy demands on the quality of housing. We found no differences between the groups as to ownership of living place, but the survivors of both sexes lived together with fewer persons and had more housing space than their controls. The survivors of both genders have almost similar housing expenditures as their controls. We were not able to find any other studies that have explored the housing aspect on cancer survivors.
The survivors in our sample did not have poorer economy than the controls according to household income and housing expenditures, although it is noteworthy that approximately 25% of both survivors and controls stated that they had financial worries last year. These findings have to be interpreted in the economic situation in Norway for the past few years, with low unemployment rates and an almost full coverage by the national health care system for periods of sick leave.
The cancer survivors had a significantly higher prevalence of other diseases than the controls. The health status of cancer survivors has been studied before, and its association to work performance has recently been examined in a qualitative study by Main et al. [15] from the USA. They found that decisions about returning to work in 29 survivors with a broad range of socio-economic background and primary cancer site were influenced by health status and symptom burden. Bednarek and Bradley [3, page 135], also from the USA, stated as a conclusion of their study: “some encouraging evidence that cancer may not deter patients continuing to work nor affect the quality of the retirement experience for those who decide to leave the labor force”. From Canada, Maunsell et al. [17] found no statistically significant difference between breast cancer survivors 3 years after diagnosis and a random sample of matched women without cancer concerning their work status. Those and other studies [3–7, 9, 23] support optimism concerning returning to work after primary cancer treatment in a similar way as the result of our study, even if our cancer survivors experience some poorer health status than the healthy population.
When work efforts of survivors are carried out in spite of disease and lower working capacity, it raises the question if this will take its toll in the long run. Only longitudinal studies can elucidate if cancer survivors will stay at work as long as controls. A recent study of long-term testicular cancer survivors from our group showed that at a mean age of 45 years and 11 years follow-up, 89% of the survivors were in paid work, which was significantly more than in a matched male population sample [9].
Increasing follow-up time after primary treatment was associated with less diseases in women, which seems to be a rather obvious finding. Female survivors reported poorer physical work capacity due to cancer than males both at short-term and long-term follow-up. This could be due to the significantly higher mean age in the female survivors.
We can easily imagine that having been treated for cancer could lead to a reduction of social participation. The present study showed similar social participation by cancer survivors as by controls. In fact, the proportion of those with enough friends was significantly higher in our female cancer survivors compared to controls, and there is a trend towards a higher proportion of close friends among male survivors. We can conclude that survivors at work did not differ from their controls as to social participation.
The protection hypothesis holds that work represents a protection against poor living condition and social exclusion because having a regular income is highly correlated with the capacity to meet one’s financial obligations [13, 15, 17, 19, 23, 26]. When we found few significant differences in the living conditions of cancer survivors and controls, we interpreted this result as a consequence of the significance of holding a job.
Steiner et al. [23] stated that inability to return to work after cancer treatment could have crucial consequences for the living conditions of survivors and their families. Having had cancer seems to improve re-evaluation of life’s themes as to the meaning of life, tolerance, motivation and relevance of work, retirement, self-accommodations and changing responsibility [15].
Strength and weaknesses
Our study focused on living condition in a relatively large sample of cancer survivors, restricted to three common cancer forms and their age-, gender- and municipality-matched controls. We used questions from established instruments for measurement of work situation and living condition. In our sample the female cancer survivors were diagnostically homogeneous because they all had breast cancer. Due to the epidemiology of cancer in men and due to age-dependent selection bias, we had to base the male sample on two diagnoses, where men with testicular cancer are younger and those with prostate cancer are older. This heterogeneity can be seen as a weakness, as well as the relative small sample sizes of the two forms of male cancer.
A response rate of approximately 60% for a questionnaire study of cancer survivors is not optimal, but patients treated at our hospital are exposed to multiple follow-up investigations, particularly those with breast and testicular cancer. This could explain the somewhat low response rate among these patients. The 45% response rate in controls from the general population without reminder is considered as acceptable.
As the attrition analysis of non-compliant and compliant cancer survivors did not show any significant differences as to age, gender, or cancer parameters, we think that our findings are generalizable to the cancer survivor sample without relapse and without major somatic symptoms within the age below 57 years at primary treatment and who later join the labour force. We have no data on the non-attending persons of the general population, and therefore no opportunity to analyse the non-compliant part of that sample. However, we cannot reject that selection bias is valid in our samples. Although the diagnosis and treatments parameters did not differ between the complying and non-complying cancer survivors, there is a definite risk that those with the most favourable living conditions have responded to our request. The same may be true for the controls, namely, that those less favourable and out of work are non-compliers. Any differences compared to other studies may be due to differences in study design or measurements, but may also cause the difference between different cohorts and different countries.
Conclusion
In general, our results show that in spite of poorer health, tumour-free survivors after breast, testicular, and prostate cancer participating in the work force report mostly similar living conditions as their matched controls. The protection hypothesis of holding jobs as a precondition for normal living conditions was therefore confirmed in our study of cancer survivors with favourable oncological outcome. In our view these findings can raise a certain optimism concerning cancer survivors’ living conditions if they are able to join the work force even for a short time or between the times of treatments. However, work with impaired mental and physical work capacity and more diseases could have negative consequences on work performance of cancer survivors in the long run, and health care professionals therefore should focus on these issues during follow-up programmes.
References
Arozullah AM, Calhoun EA, Wolf M, Finley DK, Fitzner KA, Heckinger EA, Gorby NS, Schumack GT, Bennett CL (2004) The financial burden of cancer: estimate from a study of insured women with breast cancer. J Support Oncol 3:271–278
Atkinson T, Cantillon B, Marlier E, Nolan B (2002) Social indicators. The EU and social inclusion. Oxford University Press, Oxford
Bednarek HL, Bradley CJ (2005) Work and retirement after cancer diagnosis. Res Nurs Health 28:126–135
Bradley CJ, Bednarek H (2002) Employment patterns of long-term cancer survivors. Psychooncology 11:188–198
Bradley CJ, Bednarek HL, Neumark D (2002) Breast cancer and women’s labour supply. Health Serv Res 37:1309–1328
Bradley CJ, Bednarek HL, Neumark D (2002) Breast cancer survival, work, and earnings. J Health Econ 21:757–779
Bradley CJ, Bednarek HL, Neumark D, Schemk M (2005) Short-term effect of breast cancer on labor market attachment: result from a longitudinal study. Health Econ 24:137–160
Cancer Registry of Norway (2002) Cancer in Norway 2000. Cancer Registry of Norway, Institute of Population-based Cancer Research, Oslo
Dahl A, Haaland CF, Mykletun A, Bremnes R, Dahl O, Klepp O, Wist E, Fosså SD (2005) Study of anxiety disorder and depression in long-term survivors of testicular cancer. J Clin Oncol 10:2389–2395
Erikson R, Goldtorpe JH (1992) The constant flux. A study of class mobility in industrial societies. Clarendon, Oxford
Krokstad S, Westin S (2002) Health inequalities by socioeconomics status among men in the Nord-Trøndelag health study, Norway. Scan J Public Health 30:113–124
Kunst AE, Mackenbach JP (1994) Measuring socioeconomic inequalities in health. WHO Regional Office for Europe, Copenhagen
Kåresen R, Langmark F (2000) Kreftopererte kvinners psykiske, sosiale og økonomiske forhold (in Norwegian). Tidskr Nor Lægeforen 120:2741–2748
Lauzier S, Mausell E, De Koninck M, Drolet M, Hébert-Croteau N, Robert J (2005) Conceptualization and sources of costs from breast cancer: findings from patient and caregiver focus groups. Psychooncology 14:351–360
Main DS, Nowels CT, Cavender TA, Etschamaier M, Steiner JF (2005) A qualitative study of work and work return in cancer survivors. Pschooncology 14:992–1004. DOI 10.1002/pon.913
Maunsell E, Brisson C, Dubois L, Lauzier S, Fraser A (1999) Work problems after breast cancer: an exploratory qualitative study. Psychooncology 8:467–473
Maunsell E, Drolet M, Brissin J, Brisson C, Mâsse B, Deshênes L (2004) Work situation after breast cancer: result from a population-based study. J Nat Cancer Inst 24:1813–1822
Short PF, Vasey JJ, Tunceli K (2005) Employment pathways in a large cohort of adult survivors. Cancer 103:1292–1301
Spelten ER, Sprangers MAG, Verbeek JHAM (2002) Factors reported to influence the return to work of cancer survivors: a literature review. Psychooncology 11:124–131
Spelten ER, Verbeek JH, Uitterhoeve AL, Ansink AC, van der Lelie J, de Reijke TM, Kammeijer M, de Haes JC, Sprangers MA (2003) Cancer, fatigue and the return of patients to work—a prospective cohort study. Eur J Cancer 39:1562–1567
Office for National Statistics (2000) Standard occupational classification 2000 (SOC2000). http://www.statistics.gov.uk/methods-Quality/ns_sec/soc2000.asp
Statistics Norway (2005) http://www.ssb.no
Steiner JF, Cavender TA, Main DS, Bradley CJ (2004) Assessing the impact of cancer on work outcomes. What are the research needs? Cancer 8:1703–1711
Taskila-Åbrandt T, Pukkala E, Martikainen R, Karjalainen A, Hietanen A (2004) Employment status of Finnish cancer patients in 1997. Psychooncology 14:221–226
Verbeek J, Spelten E, Kammeijer M, Spangers M (2003) Return to work of cancer survivors, a prospective cohort study into quality of rehabilitation by occupational physicians. Occup Environ Med 60:352–357
Veronesi U, von Kleist, Redmond K, Costa A, Delvaux N, Freilich G, Galus A, Hudson T, McVie JG, Macnamara C, Meunir F, Pecorelli S, Serin D, CAWAC Study Group (1999) Caring about women and cancer (CAWAC): a European survey of the perspectives and experiences of women with female cancer. Eur J Cancer 12:1667–1675
Acknowledgements
This study was sponsored by a research grant from The Norwegian Foundation for Health and Rehabilitation (grant no. H0-54010/002) and a grant from the Nordic Cancer Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gudbergsson, S.B., Fosså, S.D., Borgeraas, E. et al. A comparative study of living conditions in cancer patients who have returned to work after curative treatment. Support Care Cancer 14, 1020–1029 (2006). https://doi.org/10.1007/s00520-006-0042-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-006-0042-9