Abstract
Goals of work
The aims of this study were (1) to prospectively evaluate the clinical benefits of switching from morphine to an alternative opioid, using oxycodone as first-line alternative opioid, in patients with cancer, (2) to evaluate the consistency of the clinical decision for the need to switch by comparing two hospital sites, and (3) to evaluate whether there were objective predictors that would help identify morphine non-responders who require switching to an alternative opioid and from this to construct a clinical model to predict the need to switch.
Patients and methods
One hundred eighty-six palliative care patients were prospectively recruited from two hospital sites. Responders were patients treated with morphine for more than 4 weeks with good analgesia and minimal side effects. Non-responders (switchers) were patients who had either uncontrolled pain or unacceptable side effects on morphine and therefore required an alternative opioid. The differentiation between responders and switchers was made clinically and later confirmed by objective parameters.
Results
In this prospective study 74% (138/186) had a good response to morphine (responders). One patient was lost to follow up. Twenty-five percent (47/186) did not respond to morphine. These non-responders were switched to alternative opioids (switchers). Furthermore, of 186 patients, 37 achieved a successful outcome when switched to oxycodone and an additional 4 were well controlled when switched to more than one alternative opioid. Overall successful pain control with minimal side effects was achieved in 96% (179/186) of patients. There were no significant differences in the need to switch between the two hospital sites.
Conclusions
This study has shown that proactive clinical identification and management of patients that require opioid switching is reproducible in different clinical settings and significantly improves pain control. Further studies are required to develop and test the predictive model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three quarters of cancer patients experience pain. Morphine is the strong opioid of choice for the treatment of moderate to severe cancer pain according to guidelines from the World Health Organisation. This recommendation from the WHO was derived by virtue of availability, familiarity to clinicians, established effectiveness, simplicity of administration and relative inexpensive cost. It was not based on proven therapeutic superiority over other options.
It is often assumed that all cancer pain can be relieved with morphine; the clinical response to morphine is, however, highly variable. It is thus not sensible, as has previously been assumed, to keep titrating up the dose of morphine if intolerable side effects occur in order to achieve pain control in all patients.
For the purposes of this study we defined ‘opioid switching’, a term given to the clinical practice of substituting one strong opioid with another in an attempt to achieve a better balance between analgesia and side effects. ‘Opioid rotation’ includes the group of patients that switch to an alternative opioid to improve pain and/or side effects and also includes those changing to an alternative opioid or route of administration based on patient or clinician preference. The practice of switching is gaining popularity. Robust clinical trials are needed to both evaluate this practice and elucidate the underlying mechanisms of interindividual variation in response to different opioids.
The aims of this study were (1) to prospectively evaluate the clinical benefits of switching from morphine to an alternative opioid in cancer patients, (2) to evaluate the consistency of the clinical decision for the need to switch by comparing two hospital sites [oxycodone was used as first-line switch (second line opioid) as per Royal Marsden Hospital Trust guidelines] and (3) to evaluate whether or not there were objective predictors that would help identify switchers, and from this to construct a clinical model to predict the need to switch.
It is well recognized that there are considerable difficulties in recruiting patients to palliative care studies because of issues such as attrition, consenting and fatigue. This study differs from previous studies in that it proactively recruits oncological and palliative care patients who required strong opioids and who required a switch to an alternative opioid.
Methods
This was a prospective, observational, controlled clinical study. All patients with cancer pain who required treatment with oral morphine for pain control were considered for entry into the study. Patients were recruited from two separate sites at the Royal Marsden NHS Trust, which is a tertiary cancer centre. The study was approved by the Research and Ethics committee of the Royal Marsden Hospital. All patients gave informed written consent.
The study group included responders who had been taking morphine for at least 4 weeks with good clinical benefit. Morphine non-responders/switchers were identified as those who either experienced poor pain control despite adequate titration of morphine dose or who had morphine-related side effects unresponsive to adjuvant medications. This was a subjective assessment made initially by the patient and confirmed by the clinician who then made the decision to switch the patient to an alternative opioid. At this time, the patient was entered into the study as a ‘switcher’. This was a clinical assessment made by a different clinician at each site. Before the first dose of the alternative opioid was given objective measures of the pain and side effects were made using the Modified Brief Pain Inventory score and toxicity scores.
Patients were switched to oxycodone as first line. Second-line switch was either to fentanyl or methadone. Patients with predominantly neuropathic or incident pain and patients with abnormal renal function (serum creatinine >1.5 times the upper limit of normal as per laboratory reference range) were excluded from the study.
Data collected included demographic information, opioid doses and concomitant medications. For switchers, we also recorded the criteria for changing to an alternative opioid and whether or not the switch was successful. All patients completed a Modified Brief Pain Inventory scoring pain on a numerical rating scale of 0–10. Toxicity scores were recorded on a 4-point scale as ‘not at all’, ‘a little’, ‘quite a bit’, ‘very much’. Biochemical and haematological data at the time of entry to the study were collected.
An accurate database was constructed. All data were entered and checked by at least two members of the research team. Data was then audited by the chief scientist using data mining software. Patients were withdrawn from the study if they requested to do so or if they were too ill to continue.
Data analysis
For normally distributed variables, data are described as mean±standard deviation and Student t tests were used to compare groups. For skewed variables, data are described as median (range) and Wilcoxon rank sum tests were used to compare groups. Proportions were compared using chi-square statistics.
We analysed different indices collected at the time of entry into the study. This was either at the time of being controlled on morphine for 4 weeks or more or at the time before the patients were switched to an alternative opioid. From these variables, we constructed a model to objectively predict the need to switch from morphine to an alternative opioid. The model was built by selecting variables that reached a significance of p<0.1 on univariate analysis. In addition, gender, ethnicity and age were included in the multivariate analysis. We then performed stepwise removal of those variables that failed to reach significance at p<0.1. In addition, patients were scored as to whether or not they were being treated with the following analgesic agents in addition to opioids: steroids, non-steroidal anti-inflammatory drugs or anti-neuropathic agents. The model was tested to see whether use of these other analgesic agents would affect outcome. The impact of the following biochemical variables that are known to clinically affect side effects such as confusion, nausea and drowsiness were also considered: sodium, calcium, creatinine and albumin.
Results
Response to morphine and switching
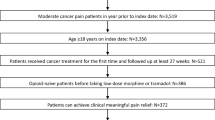
In this prospective study, 186 patients taking morphine for pain relief were recruited. Of these 186 patients, 138 had a good response to morphine (responders). These responders were compared with 48 non-responders (switchers) who required an alternative opioid. Table 1 describes the baseline characteristics for each group. For the 48 non-responders, switching was successful in 87% (41/47) of cases; outcome was not known for one patient. A single switch to oxycodone resulted in a successful outcome in 79% (37/47) of the non-responders. Two non-responders required two switches and 2 required three switches before good clinical outcome was achieved. Overall ‘switching’, where indicated, resulted in a good clinical outcome in 96% (179/186) of the patients recruited to this study (Fig. 1).
Data were analysed for 186 patients; 48 patients were switched. Switching was considered effective in 41 (87%) of 47 patients; 37 achieved analgesia and tolerable side effects with one switch, 2 with two switches and 2 patients required three switches. For 1 patient, outcome of switch was not known. Outcome was successful for 96% of patients
The most common reasons for switching to an alternative opioid were pain, confusion and drowsiness, nightmares and nausea (Table 2).
Difference in pain control and side effects between switchers and controls
Having decided on clinical grounds that a patient was not responsive to morphine because of inadequate analgesia or intolerable side effects (as reported by the patients and assessed by the clinician), we analysed patients’ pain and side effects scores to see if there was objective evidence (using the Brief Pain Inventory (BPI) and toxicity scores) to correlate with the clinical picture and the need to switch. Switchers, compared with morphine responders, had higher pain scores and obtained less pain relief from morphine [average pain, switchers 5 (0–10), responders 2 (0–8), z=−4.95, p<0.0001; worst pain, switchers 8 (2–10), responders 5 (0–10), z=−4.92, p<0.0001; pain relief, switchers 60% (0–100%), responders 80% (20–100%), z=−5.25, p<0.0001]. Switchers experienced more side effects [moderate/severe confusion (score 2 or 3), 25/48 vs. 16/138, χ2=34.0, p<0.0001; severe drowsiness (score 3), 27/48 vs. 13/138, χ2=46.3, p<0.0001; nightmares (score 2 or 3), 10/48 vs. 4/134, χ2 16.5, p<0.0001; severe nausea (score 3), 9/48 vs. 8/138, χ2=7.2, p<0.05]. The strongest association with switching was patients who were both confused and had a drowsiness score >2 (p<0.0001).
Comparison between centres on the decision to switch
The key findings of this study are that the process of identification and management of patients who require switching (i.e. do not respond to morphine or suffer intolerable side effects from it) is reproducible and successful. This is in spite of different case mixes and sites of tumour types. It is also reproducible if staff in different units use one set of guidelines. No difference in the reason for switching was found between the two hospital sites. Overall, patients recruited at one site were younger (55.5±12.9 vs. 62.6±11.8 years, t=−3.49, p<0.001) whilst the other site had a higher percentage of ethnic minority patients (4/54 vs. 20/112, χ2=8.7, p<0.05). Tumour diagnoses differed between sites as determined by geographical location of departments, sarcoma was overrepresented at one site and lung cancer and haematological malignancy at the other (25/112 vs. 1/54, χ2=11.6, p<0.001; 9/112 vs. 11/54, χ2=7.5, p<0.05; 3/112 vs. 8/54, χ2=9.7, p<0.05, respectively). Paracetamol was less likely to be prescribed at one site, reflecting clinician preference (7/54 vs. 57/112, χ2=13.4, p<0.001).
Difference between short- and long-acting opioid use between switchers and morphine responders
Switchers were more likely to be treated with short-acting rather than slow-release preparations of morphine (27/48 vs. 16/138, χ2=40.0, p<0.0001). This was an expected outcome, as patients with unstable pain are more likely to be on short-acting opioids. There was no significant difference in morphine dose between groups.
Construction of a model to predict a switcher
We found seven variables that were significantly different in the group that needed to switch with a significance of p<0.1 on univariate analysis (Table 3). In addition, gender, ethnicity and age were included in the multivariate analysis. Subsequent, stepwise removal of variables that failed to reach significance at p<0.1 resulted in a model with seven independent variables reaching significance of p<0.05. The need to switch was dependant on white cell count, weight, concomitant use of 5HT3 antiemetics, beta blockers and proton pump inhibitors, tumour diagnosis of the lower gastrointestinal tract and recent chemotherapy (within 14 days) (Table 4).
Patients were scored as to whether or not they were being treated with the following analgesic agents in addition to opioid: steroids, non-steroidal anti-inflammatory drugs or anti-neuropathic agents. The model was tested to see whether use of these other analgesic agents would affect outcome. They did not reach significance on univariate analysis and did not add to the overall model on multivariate analysis. Data used in the construction of this model were data from this prospective study only.
The following biochemical variables, which are known to clinically affect side effects such as confusion, nausea and drowsiness, were also considered: sodium, calcium, creatinine and albumin. None of these variables reached a level of significance in the multivariate analysis and therefore are not included in the final model. Developing and testing of this model needs to be done in further studies.
Patients that did not respond to morphine or alternative opioids: 3% (5/186)
Additional data regarding the five patients who did not respond to switching is presented in Table 5. The review of the notes shows that 4 of 5 patients were within 1 month of their death. One patient achieved pain control after an epidural. For three patients, in retrospect, management of their pain may also have been improved with an anaesthetic regional neural blockade.
Discussion
The opioid of first choice for moderate to severe cancer pain is morphine [1]. Large interindividual variation in analgesic response to morphine has been shown in patients with cancer. For these patients, switching to an alternative opioid is becoming established clinical practice [2, 3]. Oxycodone is used as the first-line alternative opioid for patients who switch at the Royal Marsden Hospital, as per the Palliative Care Guidelines. Evidence base for the use of oxycodone as a strong opioid in cancer pain is growing [4–10]. Other alternative opioids such as fentanyl and methadone are used as first-line alternatives in other centres [2]. One of the challenges with opioid switching is the appropriate determination of an opioid dose ratio when substituting opioids; lower doses than expected, according to equivalent conversion tables, may be required. Conversion to alternative opioids, particularly to methadone, should be done with caution [2, 11].
The rationale for using oxycodone as first-line switch is that it has similar prescribing properties to morphine with short- and long-acting preparations, easy conversion ratios (oral morphine/oxycodone, 2:1) and is familiar to hospital and community palliative care staff. It also has a different metabolic pathway.
It is a well-recognised clinical practice to titrate analgesic medication using short-acting (normal release) opioids and that is why switchers were more likely to be on oramorph or sevredol compared to sustained-release preparations.
Several studies have suggested that the variability in response to morphine may be related to patient characteristics such as age [12], renal and hepatic function [13–15], concomitant medications [16], and the mechanism of pain. In this study we excluded patients with severe renal failure (creatinine level >1.5×the upper limit of normal) and patients with predominantly neuropathic pain, and recorded individual patient data for the other variables.
The reliability of switching as a true phenomenon has long been debated. We defined an opioid switch as the clinical practice of substituting one strong opioid with another in an attempt to achieve better balance between analgesia and side effects. The broader term of opioid rotation, which includes switching, but also includes changing to an alternative opioid or route of administration of the opioid, based on patient or clinician preference has been confused with the above definition of switching in some studies. This may in part account for our greater proportion of responders since the switch group is identified more precisely.
An obstacle to testing the phenomenon of switching has been a lack of an ethical model (i.e. it would be unethical to rechallenge a patient with morphine after a satisfactory outcome on an alternative opioid.) Other studies have shown some reliability in how good clinicians are at judging treatment outcomes [17]. Our study shows that 87% (41/47) morphine non-responders did improve when switched to an alternative opioid suggesting that side effects were morphine related although we accept that we could not control for all other variables. A more objective model than clinical assessment would be preferable, but it is also important that any resultant model is useful and transferable into the clinical environment.
In this study 75% (138/186) of patients had adequate analgesic control and minimal side effects using morphine. A further 22% (41/186) were controlled after switching to an alternative opioid. The overall pain control with minimal side effects was achieved in 96% (179/186) of patients recruited to the study. Employing the process of switching thus significantly improved pain control. Other heterogeneous prospective studies, which used a variety of opioids, suggest differing magnitudes of clinical improvement on switching to alternative opioids [3, 18, 19].
In our results 4 (9%) of 47 patients who switched required more than one alternative opioid before adequate pain control was achieved. This compares favourably with one prospective survey where 20% of patients needed to undergo two or more switches until a satisfactory outcome was achieved [20].
Switchers, by definition, had higher pain scores and higher side effect scores than controls. It is important to note, however, that the range of scores shows significant overlap between groups. There is variation between patients in their perception of what level of pain and/or side effects are acceptable to them. We recognize that there is an ongoing need to educate both patients regarding the levels of pain control that can now be achieved and professionals in the use of techniques such as opioid switching.
The difficulties in identifying, recruiting and retaining study patients often have the effect that palliative care evaluations, by default, are on those individuals best able to cope. In consequence, trials often lack adequate statistical power and have recruitment bias. To improve recruitment across the total patient population we identified patients from biweekly ward rounds by examining all the drug charts. These include oncology patients who had not yet had palliative care involvement.
There was no significant difference in the clinical reasons for switching patients at the two different sites of the hospital, although the professionals recruiting at the two sites were different. Clinical guidelines are important in standardising practice across different groups. Heterogeneity between studies and differing practices across centres, not only in the UK but also internationally, make progress in collating research results difficult. We have shown good correlation between our two sites in identifying the switch population and hope that this can now be extended to other centres. Collaboration with other centres will enable recruitment of the significant patient numbers that are needed to further examine the concepts around opioid switching and will help to improve the precision of a model to predict the need to switch.
Using data collected in this study, a model was constructed to predict the need to switch. The multivariate analysis produced a model with seven independent variables. These included white blood cell count, weight, 5HT3 antiemetics, beta blockers, proton pump inhibitors, tumour diagnosis of the lower gastrointestinal tract and chemotherapy within the last 14 days. The model as it stands is not more predictive than a clinical judgement. The next step in developing the model will be to include genetic and immunological data on these patients and see if this refines the model. Any resultant model will be prospectively tested on a new data set.
Although the numbers who did not respond to morphine or switching are small, retrospective review of the cases suggests that it may be worth considering these patients for a neural blockade. If a block is to be performed, time is of the essence and the procedure should not be delayed. These patients do not live long but could benefit from the intervention improving the pain control of the terminal stage of their illness.
Why is it that changing to an alternative opioid improves outcome in the majority of patients?
In this study, most patients were switched to oxycodone. Oxycodone is metabolized by the liver by means of O-demethylation to form oxymorphone in a reaction catalyzed by the enzyme cytochrome P450 2D6 (CYP2D6). It is also metabolized by N-demethylation to noroxycodone. Noroxycodone, has only weak affinity for μ-opioid receptors [21]. Oxymorphone accounts for only 10% of oxycodone metabolites, even though it is a potent analgesic. Oxycodone, but not oxymorphone, appears to be responsible for both side effects and analgesia. Morphine is metabolized into M6G and M3G. M6G toxicity does appear to be attributable to a specific opioid mechanism and is thought to be responsible for the increased drowsiness, nausea, vomiting, respiratory depression and coma that may be observed. Oxycodone in vivo is a potent μ-agonist and at least in part, its analgesic action is mediated by active metabolites [6]. Whilst most of the analgesic activity of oxycodone is mediated by μ-opioid receptors, there are data that suggest that the intrinsic antinociceptive effects of oxycodone are mediated by κ-opioid receptors, in contrast to morphine, which interacts primarily with μ-opioid receptors [5]. In addition, different opioids bind to different regions of the μ-opioid receptor and may bind with weaker affinity to other opioid receptors. Thus, genetic variation in a number of candidate genes could potentially explain the intra-individual variability in response to these two drugs. Further data is needed to explore this hypothesis.
Conclusion
Not all patients with severe cancer pain respond to morphine. This is due to either inadequate pain control or intolerable side effects. In this study, we showed that up to 25% of patients did not respond to morphine.
Of the cancer patients with pain entered into the study 75% (138/186) were morphine responders, a further 20% (37/186) responded to a single switch to oxycodone and an additional 2% (4/186) responded after switching to more than one alternative opioid. Prospectively identifying non-responders and switching them to an alternative opioid according to established guidelines thus gave an overall successful outcome (i.e. good analgesia and minimal side effects), in 96% (179/186) of patients. In the 3% that do not respond to the strategy of switching, regional neural anaesthetic blockade should be considered.
This clinical phenomenon of prospectively identifying non-responders and switching them to alternative opioids was found to be reproducible across different units.
A clinically objective model for predicting switchers has been presented. This model is in its infancy and has no use in clinical practice in its present form. It needs further development by the addition of genetic and immunological data. It will then require testing prospectively in a further patient population. Ultimately, the identification of patients with a high probability of morphine intolerance will result in those patients starting on an alternate opioid as first-line treatment minimizing frequency and duration of distress. The outcome of these further studies will inevitably challenge the perceived wisdom of morphine being the first-line treatment for moderate to severe pain in cancer patients.
References
World Health Organisation (1996) Cancer pain relief, 2nd edn. World Health Organisation, Geneva
Mercadante S, Casuccio A, Fulfaro F, Groff L, Boffi R, Villari P et al (2001) Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol 19(11):2898–2904
de Stoutz ND, Bruera E, Suarez-Almazor M (1995) Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage 10(5):378–384
Poyhia R, Vainio A, Kalso E (1993) A review of oxycodone’s clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manage 8(2):63–67
Ross FB, Smith MT (1997) The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain 73(2):151–157
Kalso E, Vainio A, Mattila MJ, Rosenberg PH, Seppala T (1990) Morphine and oxycodone in the management of cancer pain: plasma levels determined by chemical and radioreceptor assays. Pharmacol Toxicol 67(4):322–328
Saarialho-Kere U, Mattila MJ, Seppala T (1989) Psychomotor, respiratory and neuroendocrinological effects of a mu-opioid receptor agonist (oxycodone) in healthy volunteers. Pharmacol Toxicol 65(4):252–257
Gagnon B, Bielech M, Watanabe S, Walker P, Hanson J, Bruera E (1999) The use of intermittent subcutaneous injections of oxycodone for opioid rotation in patients with cancer pain. Support Care Cancer 7:265–270
Kalso E, Vainio A (1990) Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther 47:639–646
Kalso E, Poyhia R, Onnela P, Linko K, Tigerstedt I, Tammisto T (1991) Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand 35:642–646
Mercadante S (1999) Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 86(9):1856–1866
Riley J, Ross JR, Rutter D, Shah S, Gwilliam B, Wells AU et al (2004) A retrospective study of the association between haematological and biochemical parameters and morphine intolerance in patients with cancer pain. Palliat Med 18(1):19–24
D’Honneur G, Gilton A, Sandouk P, Scherrmann M, Duvaldestin P (1994) Plasma and cerebrospinal fluid concentrations of morphine and morphine gluronides after oral morphine. The influence of renal failure. Anesthesiology 81:87–93
Farrell A, Rich A (2000) Analgesic use in patients with renal failure. Eur J Palliat Care 7(6):201–205
Hasselstrom J, Eriksson S, Persson A, Rane A, Svensson JO, Sawe J (1990) The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol 29(3):289–297
Lesage P, Portenoy RK (1999) Trends in cancer pain management. Cancer Control 6(2):136–145
Regan J, Yarnold J, Jones PW, Cooke NT (1991) Palliation and life quality in lung cancer; how good are clinicians at judging treatment outcome? Br J Cancer 64(2):396–400
Quigley C (2004) Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev 3:CD004847
Bruera E, Franco JJ, Maltoni M, Watanabe S, Suarez-Almazor M (1995) Changing pattern of agitated impaired mental status in patients with advanced cancer: association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage 10(4):287–291
Cherny NJ, Chang V, Frager G, Ingham JM, Tiseo PJ, Popp B et al (1995) Opioid pharmacotherapy in the management of cancer pain: a survey of strategies used by pain physicians for the selection of analgesic drugs and routes of administration. Cancer 76(7):1283–1293
Heiskanen T, Olkkola KT, Kalso E (1998) Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacol Ther 64(6):603–611
Acknowledgements
This study was supported by the Paul Hamlyn Foundation and the Edmund J. Safra Philanthropic Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riley, J., Ross, J.R., Rutter, D. et al. No pain relief from morphine?. Support Care Cancer 14, 56–64 (2006). https://doi.org/10.1007/s00520-005-0843-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0843-2