Abstract
Goals of work
The aims of the present study were to verify whether an innovative therapeutic strategy for the treatment of mild-moderate chronic cancer pain, passing directly from step I to step III of the WHO analgesic ladder, is more effective than the traditional three-step strategy and to evaluate the tolerability and therapeutic index in both strategies.
Methods
Patients aged 18 years or older with multiple viscera or bone metastases or with locally advanced disease were randomized. Pain intensity was assessed using a 0–10 numerical rating scale based on four questions selected from the validated Italian version of the Brief Pain Inventory. Treatment-specific variables and other symptoms were recorded at baseline up to a maximum follow-up of 90 days per patient.
Results
Fifty-four patients were randomized onto the study, and pain intensity was assessed over a period of 2,649 days. The innovative treatment presented a statistically significant advantage over the traditional strategy in terms of the percentage of days with worst pain ≥5 (22.8 vs 28.6%, p<0.001) and ≥7 (8.6 vs 11.2%, p=0.023). Grades 3 and 4 anorexia and constipation were more frequently reported in the innovative strategy arm, although prophylactic laxative therapy was used less in this setting.
Conclusions
Our preliminary data would seem to suggest that a direct move to the third step of the WHO analgesic ladder is feasible and could reduce some pain scores but also requires careful management of side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is universally known that the WHO analgesic ladder, developed during the period 1986–1990 [26, 27] as an educational tool and aid to clinical practice for the treatment of chronic cancer pain (CCP) and updated in 1996 [28], is composed of three steps of pharmacological therapy. Treatment involves the use of nonsteroidal anti-inflammatory drugs (NSAIDs) with or without adjuvants in step I, opioids for mild-moderate pain (e.g. weak opioids) with or without NSAIDs and adjuvants in step II, and opioids for moderate-severe pain (e.g. strong opioids) with or without NSAIDs and adjuvants in step III.
Despite the widespread use of the ladder, the level of analgesia reported by different authors in various patient populations is still not optimal [3, 5, 6, 23]. This may be due to barriers that hinder the correct and effective use of all the available drugs, due to the fact that the WHO guidelines are not correctly applied, or because chronic cancer pain is more difficult to treat than is generally believed. It has been reported that some works, especially case studies, based on the WHO ladder, suffer from methodological limitations [1, 12, 24, 29].
As weaknesses have emerged in the process of quality evaluation of the WHO analgesic ladder, some features of this method have come under discussion. In particular, some authors have questioned the real usefulness of step II, proposing the use of strong opioids when NSAIDs become ineffective [2, 4, 8, 9, 14, 15, 20, 25].
There are several potential reasons for this: the non-usefulness of step II, especially if it results in an unjustified delay in initiating step III therapy; strict regulations governing the use of step III agents; evidence of the superimposability of efficacy of steps I and II drugs in meta-analyses; and better therapeutic index of low-dose strong opioids compared to high-dose weak opioids [4, 8, 9, 14, 15, 20, 25]. However, there is still much discussion about the rejection of guidelines which have greatly improved the treatment of chronic cancer pain in clinical practice [10, 16].
The main aim of the present study was to verify whether an innovative two-step therapeutic strategy is more effective than the conventional three-step approach. For this reason, step I patients who would normally have proceeded to step II treatment were randomized into two arms; arm A received the conventional three-step treatment and arm B patients were administered the innovative two-step treatment (steps I and III only).
Materials and methods
Eligibility criteria were as follows: age ≥18 years; written informed consent; mild-moderate CCP, with an indication for weak opioid therapy according to WHO ladder guidelines; no previous or ongoing treatment with opioids; locoregional progression or metastatic disease. Patients were also required to be able to understand Italian and have sufficient cognitive function to comprehend the researcher’s requests.
During a 24-month period, patients with moderate pain (5–6 intensity rating on a 0–10 numerical scale for which WHO guidelines recommend treatment with opioids for mild-moderate pain), 54 patients with NSAID-resistant mild pain, and those whose pain was deemed by doctors to require treatment with opioids were enrolled onto the study. The subdivision in terms of pain intensity (0–4 mild, 5–6 moderate, and 7–10 severe) was chosen as the most appropriate on the basis of an ad hoc study that evaluated different cut-off levels to identify three levels of pain intensity [22].
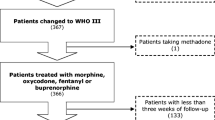
Patients were randomized to be treated, after ineffective therapy with NSAIDs, according to a conventional strategy with step II drugs or to an innovative strategy initiating with step III drugs. In the conventional arm, treatment was maintained for as long as it was considered effective and well tolerated. When this became insufficient, step III drug treatment was initiated (Fig. 1).
In the study protocol, no restrictions were placed on the use of a specific drug within each drug step.
Researchers were not limited in their use of adjuvant drugs when pain was not sufficiently controlled by analgesic treatments, in the event of side effects from analgesic therapy, or for pain typologies that were not completely responsive to opioids.
Upon study entry, the site, etiology, and physiopathology of the pain were registered. This included pain intensity, which was evaluated each day using a 0–10 numerical rating scale (NRS) (0=no pain, 10=strongest pain imaginable) based on four questions selected from the validated Italian version of the Brief Pain Inventory.
Other symptoms were recorded on the basis of a five-step severity scale ranging from 0 to 4 (0=none, 1=mild, 2=moderate, 3=severe, 4=very severe) and comprised anorexia, nausea, vomiting, other gastrointestinal symptoms, constipation, pruritus, sweating, neuropsychiatric disturbances, and urinary problems. These symptoms, whose origin, i.e. cancer or therapy, is virtually impossible to determine, were chosen because they could potentially be correlated with opioid treatment.
The degree of patient satisfaction with regard to the analgesic effect obtained was registered on a weekly basis using a five-step scale ranging from “very satisfied” to “very dissatisfied.”
The use of coanalgesics, adjuvants, and other treatments was recorded each day using a yes/no response technique. This was also used for the presence and intensity of other symptoms, which were considered potential side effects of the antipain treatments. Daily follow-up lasted for 3 months, as reported by other authors [7]. Patients were monitored either telephonically or by visits to the Hospice Outpatients Clinic. At least one assessment per patient was made each week.
The study protocol was approved by the institutional review board of each participating center. Patients’ signed informed consent was obtained before assignment to treatment, and all procedures were carried out in accordance with the Helsinki Declaration of the World Medical Association.
Statistical analysis
Sample size was determined a priori during the planning of the study, with therapeutic efficacy as the primary end point.
Unfortunately, after 24-month recruitment, the protocol committee decided to close the study due to a poor accrual rate, as only 54 evaluable patients had been enrolled during that period. Despite the low statistical power to identify relatively small differences in efficacy variables, the authors nevertheless decided to present their results because they were obtained from a randomized study and raised several important issues.
Analysis of the tolerability of the two strategies (defined on the basis of the presence or absence of symptoms probably correlated to treatment with analgesics and adjuvants) was planned in advance as a secondary aim with explorative intent. Results were analysed according to the intent-to-treat principle, i.e. patients were evaluated on the basis of their assigned therapy [11].
Statistical comparisons between the two groups in terms of therapeutic efficacy and tolerability data were performed using chi-square tests. All p values were based on two-sided testing, and statistical analyses were carried out with SAS v. 8.02 [21].
Results
Over 24 months, a total of 54 patients were entered onto the randomized trial. Of these, 24 underwent conventional treatment and 30 were given innovative therapy.
Patient and disease characteristics are reported in Table 1. Pain typology and the degree of patient satisfaction with regard to analgesic treatment administered are described in Table 2.
At the time of randomization, all patients were undergoing treatment with NSAIDs and adjuvants, and none had previously been administered with opioids. In a five-step satisfaction scale (from very satisfied to very dissatisfied), baseline analysis showed that 85.7% of patients in the conventional arm A and 86.2% in the innovative arm B were either dissatisfied or very dissatisfied.
The group of 24 arm A patients was globally treated for 1,400 days, whereas the group of 30 arm B patients received treatment for 1,249 days. Mild, moderate, and severe pain were observed, at admission, in 12.5, 66.7, and 20.8% of arm A patients and 23.3, 63.4, and 13.3% of arm B patients, respectively. The two groups at entry were well balanced in terms of pain intensity measured by the Brief Pain Inventory (BPI) (Table 3), symptoms present, and adjuvant treatments received (data not shown).
Median follow-up after randomization was 42 days. In arm A, patients received NSAIDs on 15.7% of treatment days, weak opioids on 70.5% of treatment days, and strong opioids on 20.4% of treatment days. Arm B patients underwent treatment with NSAIDs, weak opioids, and strong opioids on 23.8, 8.0, and 81.4% of treatment days (one patient passed from step III to step II), respectively.
Results were evaluated each day by recording the worst, average, and least pain experienced in the last 24 h, as well as the pain present at the moment of the interview.
Data on pain intensity are reported in Table 3. The worst pain in the innovative therapy arm was ≥5 on 22.8% of days (8.6% of days with the worst pain ≥7), whereas the worst pain in the conventional arm was ≥5 on 28.6% of days (11.2% of days with the worst pain ≥7) (p<0.001). Conversely, no differences were observed in the other pain evaluation categories.
Consequently, arm A patients were dissatisfied (11.6%) or very dissatisfied (12.1%) with 10.4% of treatment days, compared to 7.9 and 0.6% of arm B patients (Table 4), with a trend in favor of arm B.
Among all the symptoms recorded (Table 5), arm A showed a generally higher number of days without symptoms and a lower number of days with symptoms of any intensity. Anorexia and constipation were the most important clinical symptoms and were both present to a greater degree in the innovative than in the conventional treatment arm: grades 3 and 4 anorexia in 7.0% of treatment days in arm A vs 13.2% of treatment days in arm B, and grades 3 and 4 constipation in 5.9% of treatment days in arm A vs 17.7% in arm B.
It is interesting to note, however, that constipation was treated in 45.6% of days in the conventional arm A vs 53.5% of days in the innovative arm B. Treatment was considered prophylactic in 40.8% of arm A treatment days and in only 5.2% of arm B treatment days. This would seem to indicate unwarranted confidence in there being less severe constipation for some new methods of administration of strong opioids, which, on the contrary, should be accompanied with prophylactic therapy in the same way as all of the other formulations of weak and strong opioids.
Overall, there were no significant differences in distribution between the two arms with regard to adjuvant treatments (data not shown).
Discussion
In 1995, Jadad and Browman’s [12] systematic review of studies evaluating the WHO analgesic ladder highlighted eight works aimed at assessing the efficacy of the ladder. A meta-analysis was not possible because the studies were case series without control groups. The review also brought to light other limitations in the quality of these studies; insufficient information was provided on pain characteristics, retrospective studies were used, articles had short or variable follow-up, and a high rate of patients was lost to follow-up. However, it should be remembered that randomized clinical studies are not always easy to conduct in clinical practice, as is shown by the present work. More recently, another systematic review of literature [19] confirmed that, since the WHO ladder was first published, only a small number of studies have been conducted to validate it, thus providing limited evidence of its efficacy. In particular, the usefulness of step II has been questioned [8, 18]. Some studies have reported superimposable efficacy and side effects for high-dose weak opioids and low-dose strong opioids [10, 16], whereas others have indicated the potential for progressing from step I to step III with no severe side effects [13, 25]. Furthermore, Minotti et al. [18] did not observe any differences in analgesic efficacy or side effects when weak opioids were administered with NSAIDs. However, a few randomized studies have been conducted in this specific area of cancer care [16, 18]. Recently, a randomized study carried out by Marinangeli et al. [15] reported greater pain relief in terminally ill cancer patients who began treatment with stronger opioids than in those whose treatment followed WHO guidelines. The same authors also highlighted the need for fewer therapy changes and observed a generally greater degree of satisfaction in patients treated with stronger opioids. Marinangeli’s study, like ours, was limited by its low statistical power, indicating the need for researchers to “pool” their resources rather than carrying out small autonomous studies whose results rarely reach statistical significance. Nevertheless, the final results of Marinangeli’s study support the use of stronger opioids as first-line treatment of pain in patients with terminal cancer.
In general, the antalgic efficacy or side effects of pain therapy are assessed after a few days’ treatment. The evaluation range in the seven studies examined in the most recent review was 1–7 days [19].
In the present study, we compared two therapeutic strategies, calculating the number of days in which a certain result and a specific level of side effects were present, to evaluate the treatment efficacy and long-term therapeutic index in a setting as similar as possible to that of clinical practice.
A recent study [17] showed that analgesic therapy administered according to the WHO ladder was considered effective in 70% of cases, satisfactory in 16%, and inadequate in 14%. In addition to pain, numerous other symptoms were present that were probably mainly ascribable to both the disease and the analgesic therapy. A greater symptomatology was present during treatment with step III analgesics, which were, however, reserved for the more advanced stages of disease. The authors attributed a correlation between the presence of symptoms and analgesic drugs to only constipation, erythema, and dry mouth.
In our study, treatment in the innovative arm led to a significant reduction in the worst pain values “in the last 24 h.” Obviously, any favorable effects observed in conventional therapy patients who progressed to step III opioids are “diluted” in terms of the overall results of the treatment arm, but this had been taken into account in the study design, in which the primary endpoint was to compare the two overall strategies and not single periods of treatment. Constipation was more frequently reported in the innovative treatment group but was less often treated in a prophylactic way. This would seem to indicate that progression from step I to step III drugs of the WHO ladder is possible, provided that side effects are carefully controlled. However, evidence of a greater manageability of new formulations of strong opioids must not result in a less scrupulous approach to the treatment of constipation, which will probably require a prophylactic approach.
The results from this study, albeit limited due to the small case series, would seem to suggest that a direct move to WHO step III drugs is feasible and could reduce some pain scores but also requires careful management of side effects.
References
American Pain Society Quality of Care Committee (1995) Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 274:1874–1880
Ashby MA, Fleming BG, Brooksbank M et al (1992) Description of a mechanistic approach to pain management in advanced cancer. Preliminary report. Pain 51:153–161
Bonica JJ (1990) Cancer pain. In: Bonica JJ (ed) The management of pain, 2nd edn. Lea & Febiger, Philadelphia, PA
Brooks DJ, Gamble W, Ahmedzai S (1995) A regional survey of opioid use by patients receiving specialist palliative care. Palliat Med 9:229–238
Cherny NI, Portenoy RK (1994) Practical issues in the management of cancer pain. In: Wall PD, Melzach R (eds) Textbook of pain. Churchill Livingstone, London, UK, pp 1437–1468
Cleeland CS, Gonin R, Hatfield AK et al (1994) Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330:592–596
Du Pen SL, Du Pen AR, Polissar N et al (1999) Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. J Clin Oncol 17:361–370
Eisemberg E, Berkey CS, Carr DB et al (1994) Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: a meta-analysis. J Clin Oncol 12:2756–2765
Freynhagen R, Zenz M, Strumpf M (1994) WHO step II: clinical reality or a didactic instrument? Schmerz 8:210–215
Grond S, Radbruch L, Menser T (1999) High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage 18:174–179
ICH Harmonised Tripartite Guideline (1998) Statistical principles for clinical trials. The European Agency for the Evaluation of Medicinal Products, London, pp 22–24
Jadad AR, Browman GP (1995) The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA 274:1870–1873
Kumar KS, Rajagopal MR, Naseema AM (2000) Intravenous morphine for emergency treatment of cancer pain. Palliat Med 14:183–188
Laudico AV (1995) Cancer pain relief: a primary health care issue in the Philippines. Cancer Pain Release 8:1–4
Marinangeli F, Ciccozzi A, Leonardis M et al (2004) Use of strong opioids in advanced cancer pain: a randomized trial. J Pain Symptom Manage 27:409–416
Mercadante S, Salvaggio L, Dardanoni G et al (1998) Dextropropoxyphene versus morphine in opioid-naive cancer patients with pain. J Pain Symptom Manage 15:76–81
Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93:247–257
Minotti V, de Angelis V, Rigetti E et al (1998) Double blind evaluation of short-term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramina in chronic cancer pain. Pain 74:133–137
Porta-Sales J, Gòmez-Batiste X, Tuca-Rodriguez A, Madrid-Juan F, Espinosa-Rojas J, Trelis-Navarro J (2003) WHO analgesic ladder-or lift? Eur J Palliat Care 10:105–109
Regnard CFB, Tempest S (1992) A guide to symptom relief in advanced cancer. Haigh and Hochland, Manchester, UK
SAS/SAT (1990) User’s Guide, version 8.02. SAS Institute, Cary, NC
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS (1995) When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61:277–284
Stjernswärd J, Teoh N (1990) The scope of the cancer pain problem. In: Foley KM, Bonica JJ, Ventafridda V, Callaway MV (eds) Advances in pain research and therapy. Raven Press, New York, NY, pp 7–12
Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F (1987) A validation study of the WHO method for cancer pain relief. Cancer 59:850–856
Vielvoye-Kerkmcer APE, Mattern C, Vitendaal MP (2000) Transdermal fentanyl in opioid-naive cancer pain patients: an open trial using transdermal fentanyl for the treatment of chronic cancer pain in opioid-naive patients and a group using codeine. J Pain Symptom Manage 19:185–192
World Health Organization (1986) Cancer pain relief. World Health Organization, Geneva, Switzerland
World Health Organization (1990) Cancer pain relief and palliative care: report of a WHO expert committee. Technical report series 804. World Health Organization, Geneva, Switzerland
World Health Organization (1996) Cancer pain relief: with a guide to opioid availability. World Health Organization, Geneva, Switzerland
Zech DFJ, Grond S, Lynch J, Hertel D, Lehmann KA (1995) Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain 63:65–76
Acknowledgements
The co-authorship of all the following group members is acknowledged: Laura Fabbri (Palliative Care Unit, Forlimpopoli Hospital, Forlimpopoli), Oriana Nanni (Unit of Biostatistics and Clinical Trials, Istituto Oncologico Romagnolo, Forlì), Paola Raulli (Palliative Care Unit, Bassini Hospital, Cinisello Balsamo), Barbara Poggi and Francesca Fochessati (Department of Oncology, Infermi Hospital, Rimini), Donatella Giannunzio and Maria Lucia Barbagallo (Palliative Care Unit, Azienda Istituti Ospitalieri, Cremona), Vincenzo Minotti and Maura Betti (Department of Oncology, Monteluce Polyclinic, Perugia), Stefano Giordani (Hospice-ADO Casa della Solidarietà, Ferrara), and Elena Piazza, Roberto Scapaticci, and Sabrina Ferrario (Department of Oncology, Luigi Sacco Hospital, Milan). We thank Gráinne Tierney for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maltoni, M., Scarpi, E., Modonesi, C. et al. A validation study of the WHO analgesic ladder: a two-step vs three-step strategy. Support Care Cancer 13, 888–894 (2005). https://doi.org/10.1007/s00520-005-0807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0807-6