Abstract
Goal of work
This study investigated changes in psychological adjustment and quality of life among breast cancer patients following completion of radiation therapy.
Patients and methods
Ninety-four patients completed measures of depressed mood, anxiety, and quality of life via interview at five time points: the end of radiation therapy, 2 weeks posttreatment, the first radiation oncology follow-up appointment (4–6 weeks after treatment), 3 months posttreatment, and 6 months posttreatment.
Main results
At the conclusion of radiation treatment, participants reported elevated levels of depression, low levels of anxiety, and diminished quality of life. By 2 weeks posttreatment, depression decreased significantly and overall quality of life improved significantly, as well as quality of life in the specific FACT-B domains of Physical and Functional Well Being and the Breast Cancer Subscale. Following that time, the only significant change involved further improvement in breast-cancer-specific concerns.
Conclusions
Results suggest that the primary psychological changes associated with ending breast cancer treatment occur quickly following the conclusion of treatment. Thereafter, psychological status appears to stabilize. The implications of these findings for treatment and directions for future research are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 215,000 women will be diagnosed with breast cancer in the USA in 2004 [1], a disease that will affect them physically and psychologically. Due to earlier detection through mammography screening and advances in the treatment of breast cancer, the overall 5-year survival rate for breast cancer patients is currently at least 85% [32], and for those diagnosed with localized disease, the survival rate is 97% [1]. The high survival rate and the new conceptualization of cancer as a chronic disease [38] raise concern for quality of survivorship among women with breast cancer.
Much research has focused on the psychological experience of breast cancer patients at diagnosis and early in treatment [5, 13, 21, 29]. A meta-analytic review of 58 studies found that, compared to a healthy population, cancer patients showed somewhat higher levels of depression [37]. Among factors that are associated with adjustment to cancer, duration of time since diagnosis is positively related to adjustment and quality of life [12, 19], regardless of the type of surgery performed [16].
While psychological distress has been shown to be prevalent at diagnosis and during treatment, most studies show that distress declines within a year following treatment [18, 24]. Some research, however, shows that functional disruption among patients who completed radiation treatment extends far longer [39].
Consistent with evidence that distress diminishes over time, some evidence suggests that cancer survivors experience quality of life equivalent to or even enhanced relative to nonclinical populations [11, 19]. Overall, survivors of breast cancer rate their long-term quality of life as fairly high, and many cite positive changes as a result of having experienced cancer [27]. Furthermore, a study of breast cancer survivors (average of 5 years after diagnosis) demonstrated higher quality of life scores as compared to women without cancer on items assessing hopefulness, sense of purpose in life, and positive spiritual changes [12].
Although there is a substantial amount of psychological research conducted around the time of breast cancer diagnosis and at long-term follow-up, more information is needed about the impact of the end of treatment on patients’ lives. Several longitudinal studies [7, 26] suggest that anxiety and depression increase for many cancer patients after the cessation of treatment. Reasons for this seemingly paradoxical response to the end of treatment include the loss of the medical “safety net,” the loss of treatment as a form of “active coping,” diminished support of family and friends, and fear of recurrence [7, 29]. Anticipatory anxiety may be specifically associated with follow-up medical appointments [2, 23, 26, 29]. The so-called “return to normalcy” after treatment can be stressful or challenging [2]. Documentation of the psychological impact of the end of treatment is lacking in the literature for several reasons. First, when longitudinal follow-up is included, assessments are typically spaced at regular intervals (3, 6, and 12 months) from the time of diagnosis, missing the impact of specific events (e.g., the initiation of chemotherapy, the conclusion of treatment) [27]. Second, research investigating psychological status in survivors has mainly focused on either the impact of treatment side effects [28] or a survivor’s long-term outcomes, often several years after treatment conclusion [11, 15, 19].
Given the importance of documenting the experience of survivorship, the purpose of this study was to investigate levels of psychological distress and quality of life after the conclusion of treatment among breast cancer patients completing radiation therapy. The present study investigated the following:
-
(1)
What levels of depression, anxiety, and quality of life characterize breast cancer patients at the end of radiation treatment?
-
(2)
How levels of depression, anxiety, and quality of life change during the first 6 months after breast cancer treatment?
We hypothesized that breast cancer patients would demonstrate increased distress, as measured by elevations in depressed mood and anxiety, and diminished quality of life at the end of treatment. We also hypothesized that further increases in distress would be observed in anticipation of the first medical follow-up appointment, with subsequent improvement in distress and quality of life.
Patients and methods
Sample
The study sample was drawn from the radiation oncology practice at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine. We recruited women who (1) had stage 0, I, II, or III breast cancer, (2) were completing their last week of radiation treatment, (3) were not scheduled to undergo hospital-based treatment (such as surgery or chemotherapy) after radiation therapy, and (4) were able to speak and read English. Eligible patients were provided information about the study by their radiation oncologist and/or staff members and referred to the study coordinator. Women with the following characteristics were ineligible: (1) stage IV metastatic breast cancer; (2) a prior history of cancer (except for basal cell carcinoma); (3) inability to complete written questionnaires or telephone interviews; or (4) inability to provide informed consent. The study was approved by the Washington University Institutional Review Board, and all participants gave informed consent.
Measures
Sociodemographic measures
The following sociodemographic measures were assessed: age, race, marital status, number of children at home, highest level of education, employment status, and income.
Medical variables
The following medical variables were abstracted from patients’ charts: cancer stage, chemotherapy status, hormonal therapy status, and type of surgery. Those with more than one surgery were counted in the group representing their most invasive surgery.
Psychological variables
Depression
The Center for Epidemiological Studies-Depression scale (CES-D) is a 20-item measure of depressive symptomatology [31]. The reliability and validity of this scale were established in general and clinical psychiatric populations [31, 41], as well as with breast cancer patients [20]. Higher scores on the CES-D indicate greater degree of depressive symptomatology. The established norm for the CES-D for adults is X=9.25, based on a sample of 2,514 healthy men and women (59% female), ranging in age between 18 and 65 [31]. A score of 16 or above (of 60 possible points) suggests clinically significant depressive symptoms [9, 40].
Anxiety
The State Trait Anxiety Inventory (STAI), a measure of both state and trait anxiety, has demonstrated validity and reliability [34]. For this study, only the State Subscale was used, consisting of 20 statements that assess current or situational anxiety. Higher scores on the state anxiety subscale indicate greater levels of state anxiety. The established norm for healthy females (based on a sample of 451 women, ranging in age from 19 to 69 years) is X=35.20, whereas the norm for medical/surgical patients (based on 161 hospitalized male patients with an average age of 55 years) is X=42.68 [33]. The STAI has been used in over 3,000 studies [34], including studies of breast cancer patients [17, 30].
Quality of life
The Functional Assessment of Cancer Therapy-Breast (FACT-B) is a reliable, valid 36-item, self-report measure designed for use with breast cancer patients to assess multidimensional quality of life, with demonstrated sensitivity to changes over time [4]. The subscales of the FACT-B assess Physical Well Being, Social/Family Well Being, Emotional Well Being, Functional Well Being, and the Breast Cancer Subscale reflecting breast-cancer-specific concerns. The FACT-B is coded so that higher scores reflect better quality of life, both in the overall score and in specific subscales. The normative value for the FACT-B total score is X=112.8, based on a sample of 295 women with breast cancer (age range=28–86) [4].
Procedures
All eligible patients who agreed to participate entered the study by completing written questionnaires in the waiting area of Radiation Oncology during their last week of radiation treatment. These questionnaires focused on demographic and social variables. To assess changes over the 6 months following the end of treatment, participants were contacted at their home by telephone to complete depression, anxiety, and quality of life measures. The first telephone assessment occurred on the last day of radiation treatment (time 1). The second telephone interview occurred 2 weeks posttreatment (time 2). The third telephone interview was completed several days before the participant’s first radiation oncology follow-up appointment, which typically occurred 4–6 weeks after the end of treatment (time 3). The fourth and fifth telephone interviews occurred at 3 months (time 4) and 6 months (time 5) posttreatment, respectively. Additionally, participants consented for the investigator to review their medical records to gather relevant medical information.
Statistical analyses
Descriptive statistics were calculated for all outcome measures at each time point. A longitudinal graph (“spaghetti plot”) for each outcome measure was plotted against time in order to illustrate the trend over time for the outcome variables. These graphs are not shown here but served as an exploratory analysis for identifying a suitable model to fit the data. Based on the exploratory analysis, a longitudinal regression analysis using a random-effects model with maximum likelihood estimator was done to compare the baseline and each follow-up time point, between time 2 (2 weeks) and time 5 (6 months), as well as between any two consecutive time points. We chose a random-effects model (over repeated-measures ANOVA) because covariates can be added to the model easily and this approach has more flexibility in comparing the differences between any two time points. Time was treated as a continuous variable in order to derive the overall linear trend. Regression coefficients (β) from random-effects linear or logit models and p values were also calculated. All analyses were performed with the software STATA version 7.0.
Results
Sample characteristics
During the time of study recruitment, the radiation oncology department at the Siteman Cancer Center treated approximately 350 breast cancer patients. Of those patients, radiation oncology staff referred 144 (41%) patients to the study, based on our inclusion/exclusion criteria. Of those, 16 patients failed screening due to a prior history of cancer (n=10), additional hospital-based treatment following radiation (n=3), late referral for recruitment (n=2), or inability to complete written questionnaires (n=1). This left 128 patients who were eligible for the study. Of those, 23 declined due to lack of interest (n=10), lack of time (n=5), difficulties contacting by recruitment date (n=7), or an unidentified reason (n=1). During the course of the study, an additional 11 patients were lost to the study due to dropouts (n=5), ineligibility due to previously unreported history of cancer (n=2), ineligibility due to chemotherapy after radiation treatment (n=3), and incorrect recruitment timing (n=1). This left 94 participants, or 73% of the 128 eligible women, who participated in the study.
The mean age for participants was 55.4 years (SD 11.3, range 28–87 years). Table 1 shows the demographics for the sample. The participants were primarily Caucasian (71%), married (57%), and not employed (60%). Most (62%) had completed more than 12 years of formal education, and participants were evenly distributed across income levels. Most participants reported no children living at home (63%).
The medical characteristics of the population are outlined in Table 2. Most women (83%) had stage 1 or stage 2 disease. Most patients (80%) had lumpectomy as their surgical intervention. Slightly more than half of the sample received chemotherapy, and most of the participants (70%) were expected to receive hormonal therapy after radiation treatment.
Our sample was compared to the tumor registry of the population of patients diagnosed with breast cancer at the Siteman Cancer Center during the time period of our participant recruitment. The two groups were compared on these medical and demographic variables: cancer stage, type of cancer surgery, chemotherapy status, hormonal therapy status, age, race, and marital status. There were two significant differences between the groups—type of cancer surgery and hormonal status. Type of surgery distinguished the groups (χ2=31.6, p<0.001), with a higher proportion of mastectomy patients in the population of breast cancer patients when compared with our sample. This is expected as we recruited participants from radiation therapy, which is used routinely with lumpectomy to accomplish breast conservation. Hormonal status also distinguished the groups (χ2=11.9, p<0.001), with a higher proportion of our sample receiving hormonal therapy. Given that the rate of hormonal therapy in the study group was more consistent with what would be expected for a postmenopausal sample, the difference between the groups may reflect some underreporting in the tumor registry about planned hormonal therapy. The tumor registry data are collected at the time of diagnosis, and information concerning a treatment intervention that is typically implemented after all other adjuvant treatment is completed may not be as accurate. None of the other variables were statistically different between the groups, suggesting that our sample is generally representative of the larger population.
Psychological status at the end of treatment
Mean scores for the psychological measures are given in Table 3. At the end of treatment (time 1), the mean CES-D score across all participants was 12.9 (SD 11.0), which is significantly higher (t=3.16, p<0.01) than the established norm for adults (X=9.25) [31]. For 30% (n=28) of the participants, scores on the CES-D were equal to or greater than 16, the cutoff score for clinically significant symptoms of depression.
At the end of treatment, the mean state anxiety score across all participants was 33.5, which is significantly below (t=−3.23, p<0.01) the norm for healthy adults (X=35.20), as well as below (t=−8.08, p<0.001) the mean (X=42.68) for medical/surgical patients [33]. Scores of 36% (n=34) of the participants exceeded the norm for healthy adults.
The mean overall quality of life composite score on the FACT-B at the end of treatment was 105.7 (SD 20.3), which is significantly below (t=−2.95, p<0.01) the norm (X=112.8) for breast cancer patients [4]. Scores of 55% (n=52) of participants fell below the normative score. Only two FACT-B subscale scores were different from established norms—Physical Well Being (norm X=22.1), which was lower in the present sample (t=−3.26, p<0.01), and Emotional Well Being (norm X=16.3), which was higher in the present sample (t=7.93, p<0.001).
Changes in psychological status over time
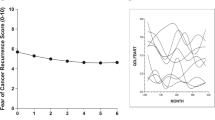
Mean depression scores decreased significantly over time (β=−0.51, t=−2.55, p=0.011) (see Fig. 1). Examination of the changes in depression scores indicated that a significant decrease in scores occurred within the first 2 weeks posttreatment, between time 1 and time 2 assessments (β=−2.97, t=−3.42, p=0.001) (see Table 4). There was no significant change in depression scores, however, between time 2 and time 5. At each assessment point, about one quarter of the participants was above the clinical cutoff for depression (30% at time 1, 25% at time 2, 30% at time 3, 23% at time 4, and 23% at time 5). The composition of this group varied, as only six participants (6%) of the total sample scored above the clinical cutoff at every time point.
There was no significant change in the mean state anxiety scores over time.
Quality of life scores improved significantly over time (β=1.92, t=5.95, p<0.001) (see Fig. 2). The FACT-B subscales that improved significantly were Physical Well Being (β=0.714, t=6.31, p<0.001), Functional Well Being (β=0.478, t=4.32, p<0.001), and the Breast Cancer Subscale (β=0.532, t=5.19, p<0.001). More specific examination of the timing of the change in the global quality of life scores revealed that significant change occurred between time 1 and time 2 (β=5.53, t=3.94, p=0.000), with no significant change thereafter. This pattern applied to other quality of life subscale scores as well: Physical Well Being (β=2.53, t=5.23, p<0.001), Functional Well Being (β=1.15, t=2.37, p=0.018), and Emotional Well Being (β=0.65, t=2.00, p=0.046). Finally, the Breast Cancer Subscale showed improvement over two time periods, between time 1 and time 2 (β=1.33, t=3.00, p=0.003) and between time 2 and time 3 (β=0.987, t=2.61, p=0.009). There were no significant changes after time 3.
Given the statistical difference in hormonal status between our sample and the population of breast cancer patients at the Siteman Cancer Center, the above analyses were rerun controlling for hormonal status. The beta values were virtually identical with and without hormonal status as a covariate, suggesting that the changes observed over time in the outcome measures were not affected by hormonal status.
Discussion
The improvements in psychological status seen in this study provide a bridge between the research on psychological status [6] and quality of life [4] at diagnosis or during treatment and the research on psychological status [15] and quality of life [36] among long-term (≥5 years) breast cancer survivors. Our results suggested that psychological “recovery” after cancer diagnosis and treatment occurred fairly quickly after the conclusion of treatment. This phenomenon is likely to be missed when follow-up measures are based on time since diagnosis or collected months or years after treatment ends. The psychological recovery may be due to concomitant relief from the effects of treatment, re-engagement in normal routines, and/or positive changes in survivors’ environment.
As expected, breast cancer patients reported somewhat heightened depression scores and diminished quality of life at the end of treatment. While significant changes in depression were found over time, the pattern of change was unexpected. Levels of depressed mood were highest at the end of treatment and improved significantly over the following 2 weeks. Similar results were found for quality of life, with significant improvement occurring in the first 2 weeks after treatment ended. This same pattern was observed for Physical Well Being, Functional Well Being, and the Breast Cancer Subscale. No other time increments in the course of the study yielded significant changes in the psychological status measures except for further improvement in the Breast Cancer Subscale between time 2 (2 weeks posttreatment) and time 3 (∼4–6 weeks after treatment).
Despite the overall improvement in depression scores, a noteworthy minority (∼25%) of participants scored above the clinical cutoff for depression at each time point, reflecting depressive symptoms that had not resolved over time or had worsened. Because symptoms of depression may be confounded with symptoms of disease or side effects of treatment, a depression measure that includes somatic items (as does the CES-D) may overestimate the prevalence of depression [2, 3, 35]. However, the number of patients with clinically significant depressive symptoms in this study was consistent with research indicating that 20–25% of breast cancer survivors report distress up to 2 years postsurgery [18], as well as other reports in the literature regarding psychological distress in cancer patients in the first 2 years after treatment [10, 24]. Thus, our results are consistent with previous findings regarding a subset of patients who have emotional difficulties past the time that most patients have made reasonable adjustment.
Although approximately 25% of our sample scored above the clinical cutoff for depression at each time point, repeated screening allowed us to determine that the composition of this group changed. In fact, a small minority of participants (6%) stayed above the cutoff at all time points. Because the CES-D does not yield formal diagnoses, the group above the cut off likely consists of various DSM diagnoses, such as Adjustment Disorder with Depressed Mood, Major Depressive Disorder, and others [35]. The probable inclusion of adjustment disorders, which may be less chronic, in the depressed group likely contributed to the transient nature of this group.
Contrary to expectation, participants did not report elevated levels of anxiety at the end of treatment. Instead, levels of anxiety remained fairly stable and relatively low throughout the study period. These findings ran counter to expectations and to some of the available literature [28]. For example, Holland [22] indicated that most cancer patients experience an increase in anxiety at the end of treatment. Participants’ lower levels of anxiety in this study were perhaps related to relief from completing treatment, reassurance from treatment staff, or adaptation to the role and stresses of being a cancer patient. Although we are unable to determine this from our data, it could be that any anxiety that was experienced at the time of diagnosis or at the beginning of treatment was resolved by the time we began our assessments. This would be an interesting question to explore in future research, although it may require a qualitative interview of a subsample of participants to explain this phenomenon.
We expected to see increased depression and anxiety at the third assessment, immediately prior to the first follow-up visit with the radiation oncologist. Previous research has documented this pattern [2, 23, 26, 29], and many patients in clinical practice have reported concern that problems (recurrence, etc.) might be identified at follow-up medical appointments. Instead, we saw little change at this time.
Fatigue has been a focus of study in the cancer literature recently. While we did not use a specific measure of fatigue, the Physical Well Being scale of the FACT-B includes two items that may be reflective of fatigue: “I have a lack of energy” and “I am forced to spend time in bed.” The Physical Well Being scale results did indicate significant improvement in physical state between the end of treatment and the 2-week follow-up. This time frame (2 weeks) may be too brief to depend solely upon significant resolution of fatigue secondary to radiation therapy, as this symptom generally resolves over a period of 3 months [25]. Future research may benefit from more specific assessment of physical symptoms and documentation of the timing of side effects and their resolution.
Tamoxifen has also been a focus of research in recent years. Our study sample differed from the population of breast cancer patients in our cancer center in terms of hormonal therapy status. Patients in our sample were more likely to receive hormonal therapy. Because of this difference, we cannot rule out the possibility that tamoxifen may have influenced our results. Our reanalysis of the data controlling for hormonal therapy status did not yield different results, suggesting that tamoxifen did not influence the particular results of this study, including the timing of the improvement in psychological status.
This study had several limitations. First, our referral rate for patients from Radiation Oncology staff was somewhat low, which could reflect a possible selection bias. However, most of the referrals to the study came from two of the four radiation oncologists, so the bias may have been more systematic in nature rather than related to patient presentation. Because we compared our sample to the population of breast cancer patients at our cancer center, we know that our sample was broadly representative of the larger group. Other limitations are primarily related to the exclusion criteria and consequent limitations on generalizability of the findings. By excluding patients with a prior history of cancer, the study cannot describe the adjustment of patients with previous experience with survivorship. Also, by excluding stage IV breast cancer patients, this study was not able to describe the adjustment of patients with metastatic cancer. However, with improvements in diagnosis and treatment, those completing radiation therapy for stage 0, I, II, or III breast cancer constitute the majority of breast cancer patients and, as such, are worthy of study as a group.
By focusing the assessments exclusively on the end of treatment, this study does not provide information about the psychological status of these women at the time of diagnosis or at the initiation of treatment. Thus, it was not possible to document changes in psychological status over the course of treatment nor to consider how our results compare to psychological status earlier in the cancer experience. This type of information is important for understanding the course of patients’ overall adjustment to cancer and should be addressed in future research.
Finally, by excluding patients who could not complete written questionnaires or telephone interviews, this study did not incorporate the experience of patients with low literacy or with life circumstances precluding access to a telephone. However, it should be noted that 16% of participants had not completed high school, whereas another 22% reported only a high school education. In addition, 29% of participants were from ethnic minorities (28% African American). Thus, the sample was broadly representative of variation in cultural and socioeconomic status.
Scientific and clinical implications
The results of this study suggest several avenues for future research. Because of the small sample size, we were unable to examine differences in psychological outcomes by cancer stage or other medical variables. Future studies with a larger sample would allow the opportunity to explore this important area. Because our study did not focus on specific psychiatric diagnoses, it would be useful for future research to document the prevalence and patterns of change in specific psychiatric diagnoses after treatment, particularly since it has been suggested that there is a low association between measures of depressive symptomatology (such as the CES-D) and psychiatric diagnoses [8]. Exploration of changes in psychiatric diagnoses over time could be accomplished by conducting a standardized clinical interview, such as the Structured Clinical Interview for DSM-IV (SCID [14]), for those who score above clinical thresholds on the CES-D. Finally, psychiatric/psychotherapeutic treatment was not provided as a part of this study. Future research could investigate the impact of psychological interventions on patient adjustment after treatment and determine whether early intervention improves long-term outcomes in this patient group. In order to test preventative strategies for depressive disorders, additional research would be helpful to identify reliable markers for those patients at greatest risk for the development of psychological/psychiatric disorders after treatment for cancer.
In conclusion, this study documented a positive pattern of adjustment for the 6 months following the conclusion of radiation treatment for breast cancer. The end of treatment can be a time characterized by higher rates of depressive symptoms and greater impairment in quality of life; however, significant improvement occurred in the first 2 weeks after the end of treatment. Moreover, symptoms of depression seemed to be transient, with only a small percentage of patients experiencing chronic problems with depression in the 6 months following treatment. From the clinician’s perspective, this means that most patients are psychologically healthy after treatment, and those who are distressed tend to recover fairly quickly. Information about this significant and rapid improvement may be useful for patients who are struggling with their reactions to treatment and for the health professionals who are directing their care. Clinicians may want to wait to evaluate patients until a few weeks after the end of treatment to see if initial psychological difficulties resolve spontaneously. Moreover, because of the changing composition of the group with elevated CES-D scores, repeated screening may help to distinguish between short-term psychological distress and more chronic problems with depression. If depressive symptoms persist, patients should be referred for more formal evaluation to determine whether psychosocial and/or psychiatric interventions are needed. The end of treatment and the months that follow represent an important transition in the long-term process of survivorship. Clinicians and patients will benefit from a hopeful, yet realistic expectation about the emotional challenges and benefits of life after cancer treatment.
References
American Cancer Society (2004) Cancer facts & figures: 2004. American Cancer Society, Atlanta, GA
Berard RMF (2001) Depression and anxiety in oncology: the psychiatrist’s perspective. J Clin Psychiatry 62:58–61
Bottomley A (1998) Depression in cancer patients: a literature review. Eur J Cancer Care 7:181–191
Brady MJ, Cella DF, Mo F et al (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986
Carver CS, Pozo C, Harris SD et al (1993) How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Pers Soc Psychol 65:375–390
Compas BE, Stoll MF, Thomsen AH et al (1999) Adjustment to breast cancer: age-related differences in coping and emotional distress. Breast Cancer Res Treat 54:195–203
Culver JL, Arena PL, Antoni MH et al (2002) Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics, and non-Hispanic whites. Psycho-oncol 11:495–504
Dausch BM, Compas BE, Beckjord E et al (2004) Rates and correlates of DSM-IV diagnoses in women newly diagnosed with breast cancer. J Clin Psychol Med Settings 11:159–169
Day R, Ganz PA, Constantino JP (2001) Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst 93:1615–1623
Dean C (1987) Psychiatric morbidity following mastectomy: preoperative predictors and types of illness. J Psychosom Res 31:385–392
Dorval M, Mannsell E, Deschenes L et al (1998) Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol 16:487–494
Dow KH, Ferrell BR, Leigh S et al (1996) An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat 39:261–273
Epping-Jordan JE, Compas BE, Osowiecki DM et al (1999) Psychological adjustment in breast cancer: processes of emotional distress. Health Psychol 18:315–326
First MB, Spitzer RL, Gibbon M, Williams JBW (1998) Structured clinical interview for DSM-IV axis 1 disorders-non-patient edition (SCID-I/NP, Version 2.0-8/98 revision). New York State Psychiatric Institute, New York, NY
Ganz PA, Greendale GA, Petersen L et al (2003) Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol 21:4184–4193
Ganz PA, Schag AC, Lee J et al (1992) Breast conservation versus mastectomy: is there a difference in psychological adjustment or quality of life in the year after surgery? Cancer 69:1729–1738
Gaston-Johansson F, Ohly KV, Fall-Dickson JM et al (1999) Pain, sychological distress, health status, and coping in patients with breast cancer scheduled for autotransplantation. Oncol Nurs Forum 26:1337–1345
Glanz K, Lerman C (1992) Psychosocial impact of breast cancer: a critical review. Annals Behav Med 14:204–212
Gotay CC, Muraoka MY (1998) Quality of life in long-term survivors of adult-onset cancers. J Natl Cancer Inst 90:656–667
Hann D, Winter K, Jacobsen P (1999) Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res 46:437–443
Heim E, Augustiny KF, Schaffner L et al (1993) Coping with breast cancer over time and situation. J Psychosom Res 37:523–542
Holland JC (1989) Anxiety and cancer: the patient and the family. J Clin Psychiatry 50:20–25
Holland JC (2003) Psychological care of patients: psycho-oncology’s contribution. J Clin Oncol 21:253s–265s
Irvine DM, Brown B, Crooks D et al (1991) Psychosocial adjustment in women with breast cancer. Cancer 67:1097–1117
Irvine DM, Vincent L, Graydon JE, Bubela N (1998) Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs 21:127–135
Lampic C, Wennberg A, Schill J et al (1994) Anxiety and cancer-related worry of cancer patients at routine follow-up visits. Acta Oncol 33:119–125
Leedham B, Ganz PA (1999) Psychosocial concerns and quality of life in breast cancer survivors. Cancer Investig 17:342–348
Mose S, Budischewski KM, Rahn AN et al (2001) Influence of irradiation on therapy-associated psychological distress in breast carcinoma patients. Int J Radiat Oncol Biol Phys 51:1328–1335
Moyer A, Salovey P (1996) Psychosocial sequelae of breast cancer and its treatment. Annals Behav Med 18:110–125
Poulsen B, Graversen HP, Beckmann J et al (1997) A comparative study of post-operative psychosocial function in women with primary operable breast cancer randomized to breast conservation therapy or mastectomy. Eur J Surg Oncol 23:327–334
Radloff LS (1977) The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Ries LAG, Eisner MP, Kosay CL et al (eds) (2000) SEER Cancer Statistics Review, 1973–1995. National Cancer Institute, Bethesda, MD
Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (Form Y). Mind Garden, Palo Alto, CA
Spielberger CD (1989) State-Trait Anxiety Inventory: a comprehensive bibliography. Consulting Psychologists Press, Palo Alto, CA
Stiefel F, Die Trill M, Berney A et al (2001) Depression in palliative care: a pragmatic report from the expert working group of the European association for palliative care. Support Care Cancer 9:477–488
Tomich PL, Helgeson VS (2002) Five years later: a cross-sectional comparison of breast cancer survivors with healthy women. Psycho-oncol 11:154–169
van’t Spijker A, Trijsburg RW, Duivenvoorden HJ (1997) Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosom Med 59:280–293
von Eschenbach AC (2003) NCI sets goal of eliminating suffering and death due to cancer by 2015. J Natl Med Assoc 95:637–639
Walker BL, Nail LM, Larsen L et al (1996) Concerns, affect, and cognitive disruption following completion of radiation treatment for localized breast or prostate cancer. Oncol Nurs Forum 23:1181–1187
Wenzel LB, Fairclough DL, Brady MJ et al (1999) Age-related differences in quality of life of breast carcinoma patients after treatment. Cancer 86:1768–1774
Zich JM, Attkisson CC, Greenfield TK (1990) Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med 20:259–277
Acknowledgements
This study was sponsored by the Alvin J. Siteman Cancer Center Research Development Grant Award.
We wish to acknowledge the researchers and staff who contributed to the ACT Project. We are particularly grateful to the staff in Radiation Oncology for their support of this project. Finally, we thank the women who participated in this study and taught us so much.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deshields, T., Tibbs, T., Fan, MY. et al. Ending treatment: the course of emotional adjustment and quality of life among breast cancer survivors immediately following radiation therapy. Support Care Cancer 13, 1018–1026 (2005). https://doi.org/10.1007/s00520-005-0801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0801-z