Abstract
Liver cancer is a leading cancer in Taiwan, especially in males. Transcatheter arterial chemoembolization (TACE) is a major treatment for these patients, but research examining their fatigue experiences is limited. The purposes of this longitudinal, correlational study were to identify (1) changes in fatigue, symptom distress, anxiety and depression in cancer patients across four time points during the first week of TACE treatment, and (2) factors predicting changes in fatigue across the four time points. Eligible male inpatients with liver cancer were recruited from a medical center in Taipei. Subjects (n=40) were assessed 1 day before (T1), and during days 2 (T2), 4 (T3) and 6 (T4) of TACE. Data were analyzed by descriptive statistics, Pearson’s correlations, repeated measures analysis of variance (ANOVA) and the generalized estimating equation (GEE). Subjects had mild to moderate levels of fatigue that peaked at T2, and showed a decrease at T3 and T4 but were still slightly higher than at T1. The GEE analysis showed that greater symptom distress, anxiety and depression, higher Adriamycin dosage, longer duration of previous fatigue, and less education significantly predicted fatigue changes. The results indicate that the pattern of fatigue in TACE during the first week is similar to fatigue in patients receiving chemotherapy. The results also further indicate that fatigue is associated to several factors. The causal relationships between fatigue and these related factors should be examined. Interventions targeting these factors should also be tested in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer, also known as hepatocellular carcinoma or hepatoma, is a critical health problem. It has been ranked fourth worldwide as a cause of cancer mortality [4]. In Taiwan, hepatoma was ranked first in incidence among males in the year 2000 [6], and as the top cause of death among all cancer deaths in the year 2002 [10]. Both surgical dissection and transcatheter arterial chemoembolization (TACE) have been recognized as major modalities for treating liver cancer [17, 23, 37]. TACE is a treatment that occludes blood vessels supplying tumors by using various embolizers, such as Gelfoam cubes, or powders, or by injecting chemotherapeutic drugs. Adriamycin, the chemotherapeutic agent most commonly used for TACE, is known to be an effective treatment but has major side effects. Because the route of administration of chemotherapeutic drugs by TACE differs from the usual intravenous (IV) route, patients’ experiences with Adriamycin may also differ. Clinical observations in Taiwan indicate that many patients experience fatigue during TACE, but there has been little research exploring this problem.
Fatigue has been recognized as one of the most distressing and common problems faced by patients receiving chemotherapy [1, 5, 12, 18, 20, 21, 34]. Like pain, fatigue has a negative impact on the patient’s quality of life [13, 25, 35] and daily function [11], but until recently it has received even less attention than pain [11]. Understanding patients’ fatigue experience, such as its pattern and associated factors during cancer treatment can help health-care professionals identify patients’ fatigue problems and care needs. Numerous studies have examined fatigue patterns experienced by patients receiving chemotherapy, but most of these studies have targeted breast cancer patients receiving chemotherapy [1, 2, 19, 33]. Fatigue patterns have been found to differ with various chemotherapeutic protocols [1].
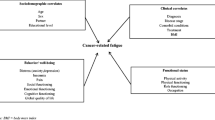
Besides characterizing fatigue patterns, related factors need to be identified to give health-care professionals a more comprehensive basis for understanding and managing cancer-related fatigue. Previous studies have shown that symptom distress [2, 18, 22, 29], and psychological distress (such as anxiety and depression) [5, 18, 22, 29] might be associated with cancer patients’ fatigue. Treatment-related factors [1, 32] and fatigue with prior chemotherapy [19] might also be related to fatigue during chemotherapy. However, to our knowledge, these factors have not yet been examined simultaneously. Furthermore, the correlates of fatigue were examined in a cross-sectional manner. In other words, factors related to fatigue were assessed at only one time point, which may not adequately capture the nature of fatigue as a continuous process.
In Taiwan, most of the research on liver cancer has targeted the effectiveness of treatment-related or basic science issues [7, 8, 16]. One study, however, directly examined patients’ fatigue problems during TACE treatment [24] and found that fatigue was the most distressing issue for patients. However, that study did not examine psychological, disease- and treatment-related factors (e.g. dose of chemotherapeutic agent). Given the high incidence of liver cancer worldwide, particularly in Asia [4], understanding patients’ fatigue experience and its related factors during TACE is crucial in helping health-care professionals provide better care.
Therefore, the aims of this study were: (1) to examine fatigue levels and patterns during the first 6 days of TACE treatment, and (2) to identify the factors that predict the changes in fatigue during the first 6 days of TACE treatment. Fatigue was assessed at four time points: 1 day before TACE treatment (T1), and during days 2 (T2), 4 (T3) and 6 (T4) of TACE. Demographic and disease-related variables (age, education, being accompanied by a family member, tumor size, duration of previous fatigue, number of previous TACE treatments, dosage of Adriamycin), symptom distress, anxiety and depression were examined as potential factors predicting longitudinal changes of fatigue measured across the four time points.
Methods
Subjects and setting
A prospective longitudinal, correlational design was used in the current study. Eligible subjects were inpatients with hepatocellular carcinoma admitted for TACE. Subjects were recruited from two gastroenterology inpatient wards in a Veterans’ Administration (VA) general hospital in northern Taiwan. The VA general hospital is also one of the leading medical centers in Taiwan for treating liver cancer patients. Thus, not only veterans, but also the general public are admitted. However, since liver cancer occurs in males and females at a ratio of 8 to 3 in Taiwan [6], and more males tend to be admitted to the VA hospital, very few female patients were recruited. Due to the limited number of women subjects (n=5), we analyzed only data from male patients.
Procedure
Before data collection, the study was evaluated and approved by the Institutional Review Board of the hospital. Informed consent was obtained from patients before they were interviewed. Data were collected at four time points: the day before TACE (T1), and on days 2, 4 and 6 of TACE (T2, T3 and T4). Each patient was interviewed and required to complete the scales at approximately the same time of day over the time period from the day before TACE. At each time point, fatigue, symptom distress, depression, and anxiety were assessed. At T1, data on demographic and disease-related variables were also collected (see below).
Instruments
Four scales were used to assess patients’ demographic and disease-related information, fatigue, symptom distress, and psychological distress: a background information form, the revised Piper Fatigue Scale (PFS) [31], the modified Symptom Distress Scale (SDS-m) [20], and the Hospital Anxiety and Depression Scale (HADS) [39], respectively. Background information included age, gender, education (years in formal school education), religion, being accompanied by a family member, tumor size, number of previous TACE treatments (none, one, two to four, five or more), Adriamycin dose this time (mg), and duration of previous fatigue (none, <6 months, ≥6 months).
The PFS [31] was used to measure level of fatigue. The PFS, one of the most commonly used scales to assess cancer patients’ fatigue level, has 22 items scored from 0 (no fatigue) to 10 (severe fatigue). As suggested by Piper [30], the level of fatigue was categorized according to score as “no fatigue” (score 0), “mild” (score 1–3), “mild to moderate” (score 4–6), and “severe” (score 7–10). Its psychometric characteristics have been demonstrated to be satisfactory [2, 31, 35]. A Chinese version of the PFS, used for this study, was rigorously translated and back-translated by the authors following the rule of instrument translation across different languages [26]. Because all subjects were hospitalized, two items, “the ability to complete your work or school activities” and “your ability to engage in sexual activity,” were deleted. The modified PFS retained 20 items. The higher the score, the greater the level of fatigue. In this study, Cronbach’s alpha for the revised PFS was 0.97.
Symptom distress was measured using the SDS-m [20, 28], which has been shown to have satisfactory psychometric characteristics for detecting cancer patients’ symptom distress [27, 28]. The original SDS is a 13-item, Likert-type scale with responses ranging from 1 (no symptom at all) to 5 (severe and can’t tolerate). The higher the score, the greater the level of symptom distress. The Chinese version of the SDS has been shown to be reliable [20]. For this study, we kept only items directly related to symptoms of physical distress, excluding three psychologically related items (outlook, concentration, restlessness) and fatigue (to avoid overlap with the dependent variable [fatigue] in this study). The final SDS used in this study (SDS-m) had nine items; Cronbach’s alpha was 0.76.
The HADS [39] was used to measure levels of anxiety and depression. The 14-item HADS has two subscales (anxiety and depression), each with seven items. Each item is scored from 0 (“not at all”) to 3 (“always”). Anxiety (depression) scores range from 0 to 21 The higher the score, the greater the level of anxiety (depression). Satisfactory psychometric characteristics have been shown for the HADS in cancer-related studies in Taiwan [9]. In this study, Cronbach’s alpha values for the anxiety and depression subscales were 0.71 and 0.66, respectively.
Data analysis
Data were analyzed by descriptive statistics, repeated-measures analysis of variance (ANOVA), Pearson’s correlation, and the generalized estimating equation (GEE). Repeated-measures ANOVA was used to examine differences in fatigue levels over the four times (T1, T2, T3, T4). For descriptive purposes, Pearson’s correlations were used to explore preliminary relationships among fatigue, symptom distress, anxiety, and depression at each time. The GEE was normalized by Liang and Zeger to extend generalized linear models to a regression setting for repeated observations within subjects [14, 38]. The GEE can appropriately determine population-averaged estimates, accounting for correlations between repeated observations [15, 38]. Independent variables used to predict changes in fatigue were demographic variables (age, education, being accompanied by a family member), disease and treatment-related variables (duration of previous fatigue, number of previous TACE treatments, Adriamycin dosage, tumor size), overall symptom distress, depression and anxiety.
Results
Subject characteristics
Of the 52 male subjects recruited, 40 completed all four assessments. Among the 12 who did not complete the study, five refused to participate and seven completed only the pre-TACE assessment, refusing to continue after TACE due to physical discomfort. Comparison of baseline data for the 40 patients who completed the study and the seven who dropped out showed no significant differences in their background characteristics, fatigue level, symptom distress, anxiety or depression.
As shown in Table 1, the mean age of the 40 male patients who completed the study was 67.38 years (SD 10.27, mode 65). The mean education level was 9.70 years (SD 5.23). Two-thirds of the subjects (67.5%) were accompanied by a family member. More than half of the patients did not have any religious belief (52.5%). Only 14 patients (35%) reported not experiencing fatigue before the current TACE. Of the 30 patients who had previously received TACE, 16 had been treated two to five times. The majority of the patients (n=27, 67.5%) were receiving 30 mg of Adriamycin for the current TACE treatment. Tumor sizes (determined by standard medical assessments during TACE) ranged from less than 1 cm to 14 cm.
Changes in fatigue, symptom distress, anxiety and depression
The levels of fatigue, symptom distress, anxiety and depression at the four time points were analyzed separately by repeated measures ANOVA (Table 2). The results of this within-group comparison showed that there were significant differences in levels of fatigue, symptom distress, and anxiety over time, but not in depression (Table 2). In general, patients had mild to moderate levels of fatigue that peaked on the second day of TACE and decreased on the fourth and sixth days (Fig. 1). Symptom distress, overall, was mild and followed a similar pattern to that of fatigue, peaking on the second day of TACE. The three most distressful symptoms across the four time points were insomnia, appetite loss, and pain. Abdominal distension, however, was perceived as the second most distressing symptom on the day before TACE for these liver cancer patients. Taken together, significant increases in fatigue and overall symptom distress were seen on the second day of TACE.
The mean scores for anxiety and depression for each assessment section, overall, were under 7, except for the anxiety level on the second day of TACE (Table 2). The pattern of depression was similar to that of fatigue, with a peak on the second day of TACE, but this increase was not statistically significant. Anxiety levels showed a significant peak on the second day of TACE treatment.
Relationships among fatigue and related variables
Relationships among fatigue and its correlates were first explored by Pearson’s correlation, and if a significant relationship was found between fatigue and any of these factors collected at any time point, the relationship between the factor and fatigue pattern was further evaluated using the GEE (T2, T3 and T4). The results of the GEE analysis revealed that, after controlling for the time factor, patients with less education, receiving a higher dose of Adriamycin, with longer duration of previous fatigue, more symptom distress, greater anxiety and greater depression levels perceived a greater level of fatigue during the first week of TACE treatment (Table 3). However, time itself did not reach the level of significance (P=0.135). These findings suggest that the changes in fatigue during the first 6 days after TACE were not related to time but were related to changes in other factors, such as symptom distress, anxiety and depression.
Discussion
This is the first study in Taiwan to examine the severity and changing pattern of fatigue and its correlates in liver cancer patients during the first week of TACE treatment. There were several important findings which are discussed below.
Overall, patients in this study perceived a mild to moderate level of fatigue across the four time points, ranging from 3.55 (SD 1.70) to 4.85 (SD 1.71). These findings are similar to those of So (4.7±1.7) who used the PFS to examine fatigue levels in Chinese patients with hematological malignancies after bone marrow transplantation [35]. In the present study, fatigue levels changed slightly over time, peaking on the second day of TACE treatment, then gradually decreasing. However, even on the sixth day of TACE (T4), the mean level of fatigue was still higher than at pretreatment (T1). These results are similar to those of Berger’s study of breast cancer patients receiving adjuvant chemotherapy [1], in which fatigue levels peaked at 48 h after chemotherapy (4.55 to 4.82 as determined by the PFS). Our findings are very similar to those of Schwartz et al. who examined chemotherapy-related fatigue [34] and found that fatigue peaked on the day after chemotherapy. These findings indicate that the pattern of fatigue in patients undergoing TACE treatment with Adriamycin is similar to the pattern found following IV administration in chemotherapy. However, more studies should be done to validate this conclusion.
Similar patterns of change throughout the first week of TACE treatment were also found for overall symptom distress, depression, and anxiety, except for a slight increase in anxiety on the sixth day. The similarity of patterns for these latter three variables and fatigue suggests that they are closely linked. Using Pearson’s correlation, we found mild to moderate to high correlations between fatigue and these three variables (Pearson’s [product moment] coefficient ranged from 0.35 to 0.66, P=0.001 to 0.0001) at each time point. This finding provides preliminary support for the possibility that these variables cluster with patient fatigue. However, in this study, we did not examine the causal relationships among fatigue and these factors. Future studies should further validate the causal relationships among these factors.
Taking the results from repeated measured ANOVA and GEE together, several important issues became apparent. First, although the change in fatigue over time was significant as analyzed by repeated measures ANOVA, time was not found to be significant in the GEE analysis, which instead showed depression, symptom distress and anxiety to be significantly related to fatigue. The results suggest that although fatigue changes over time, time itself is not the factor related to the changes in fatigue. The change in fatigue over time is related to changes in depression, symptom distress and anxiety. Second, the results of the GEE analysis generally support the major findings of previous chemotherapy-related studies. For example, depression, anxiety and symptom distress have been reported as correlates of fatigue when measured cross-sectionally [2, 3, 5, 18, 22, 29]. However, these factors have not yet received sufficient attention from clinicians. More effort, including clinical education and research, should be applied to increase the attention and ability of health-care providers to care for and manage patients’ emotional and symptom distress in order to decrease the patients’ fatigue.
Third, the GEE analysis also showed that disease and treatment-related variables, i.e. duration of fatigue before the current TACE treatment and dosage of Adriamycin, can significantly predict changes in fatigue. These results are similar to our clinical observations and provide further support for a relationship between fatigue and variables related to treatment [19, 34, 36]. The results further suggest that clinicians should be particularly aware that patients who receive higher doses of Adriamycin from TACE might be at risk of developing severe fatigue after TACE. The duration of fatigue before the current TACE is another factor not being sufficiently taken into account clinically. Assessment of the previous fatigue status before TACE would give clinicians a more comprehensive picture to allow better care and management of the fatigue of patients undergoing TACE.
Tumor size and times of receiving TACE previously were all assumed to be related to patient fatigue level, but they were not significant in this study. The dose of Adriamycin, however, was significantly related to fatigue. Since the dose of chemotherapy is basically based on the size of the tumor, chemotherapy (Adriamycin) dose and tumor size might be correlated to some degree. This result further suggests that the dose of Adriamycin may be a more sensitive reflection of patient fatigue than tumor size and the number of TACE treatments received previously. Future studies should validate these findings.
Having less education was identified by GEE analysis as a predictor of change in fatigue. The reason is unclear. Indeed, inconsistencies in the relationships between fatigue and demographic variables have previously been reported [5, 34]. Further studies should examine both issues to clarify their relationship with patient fatigue.
Although our study provided several important findings, there were a few limitations. First, since all subjects were male, the findings cannot be generalized to female liver cancer patients receiving TACE. Second, since we only examined the fatigue pattern for the first 6 days of TACE treatment, it is not clear what changes might occur over a longer period. Future studies should examine fatigue issues over a longer period. Furthermore, since symptom distress was found to be a significant predictor of changes in fatigue, individual symptoms contributing to overall symptom distress should be further examined to decrease overall fatigue in liver cancer patients. Although we did not record the pre- and post-medication used in patients receiving TACE, the severity of symptom distress might have reflected the effect of medication. However, research is still be needed to determine whether there are differences in changes in fatigue and symptom distress with different pre- and post-medication given to control the potential side effects or symptom distress, such as pain and nausea.
In conclusion, the results of this study provide a clear picture of the changing pattern of fatigue and its correlates experienced by liver cancer patients during the first 6 days of TACE treatment. In addition to a need for more longitudinal studies examining fatigue changes to validate our findings, future research should also develop and test interventions that use the predictors identified in the current study to enhance the relief of fatigue in patients undergoing TACE treatment.
References
Berger AM (1998) Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum 25:51–62
Berger AM, Higginbotham P (2000) Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum 27:1443–1448
Blesch KS, Paice JA, Wickham R, et al (1991) Correlates of fatigue in people with breast or lung cancer. Oncol Nurs Forum 18:81–87
Bosch FX, Ribes J, Borras J (1999) Epidemiology of primary liver cancer. Semin Liver Dis 19:271–285
Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH (1998) Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol 16:1689–1696
Cancer Registration System (2004) 2000 annual report. http://www.crs.cph.ntu.edu.tw
Chen JD, Liu CJ, Lee PH, et al (2004) Hepatitis B genotypes correlate with tumor recurrence after curative resection of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2:64–71
Chen MF, Jeng LB, Lee WC, Chen TC (1998) Surgical results in patients with dual hepatitis B- and C-related hepatocellular carcinoma compared with hepatitis B- or C- related hepatocellular carcinoma. Surgery 123:554–559
Chen PY, See LC, Wang CH, et al (1999) The impact of pain on the anxiety and depression of cancer patients. Formos J Med 3:373–382
Department of Health (2004) Health statistics. http://www.doh.gov.tw/static/data
Ferrell B, Grant M, Padilla G, Vermuri S, Rhiner M (1991) The experience of pain and perception of quality of life: validation of conceptual model. Hosp J 7(3):9–24
Hann DM, Jacobsen PB, Martin SC, et al (1997) Fatigue in women treated with bone marrow transplantation for breast cancer: a comparison with women with no history of cancer. Support Care Cancer 5:44–52
Hann DM, Garovoy N, Finkelstein B, et al (1999) Fatigue and quality of life in breast cancer patients undergoing autologous stem cell transplantation: a longitudinal comparative study. J Pain Symptom Manage 17:311–319
Hortom NJ, Lipsitz SR (1999) Review of software to fit generalized estimating equation regression models. Am Statistician 53:160–169
Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA (1998) Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol 147:694–703
Huang JS, Chao CC, Su TL, et al (2004) Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochem Biophys Res Commun 315:950–958
Ikeda M, Okada S, Yamamoto S, et al (2002) Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol 32:455–460
Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L (1994) The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs 17:367–378
Jacobsen PB, Hann DM, Azzarello LM, et al (1999) Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage 18:233–242
Lai YH (1998) Symptom distress and home care needs in patients receiving chemotherapy in an outpatient setting. J Nurs Res 6:279–289
Lai YH, Chang JT, Keefe FJ, et al (2003) Symptom distress, catastrophic thinking, and hope in nasopharyngeal carcinoma patients. Cancer Nurs 26:485–493
Lee YS, Tsai YF, Lai YH, Kao RH (2002) Fatigue levels and related factors in lung cancer patients during chemotherapy. Formos J Med 6:797–804
Lin DY, Lin SM, Liaw YF (1997) Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 12(9–10):S319–328
Lin ML, Tsang YM, Hwang SL (1998) Efficacy of a stress management program for patients with hepatocellular carcinoma receiving transcatheter arterial embolization. J Formos Med Assoc 97(2):113–117
Lovely MP, Miaskowski C, Dodd M (1999) Relationship between fatigue and quality of life in patients with glioblastoma multiforme. Oncol Nurs Forum 26:921–925
Marin G, Marin BV (1991) Research with Hispanic populations. Applied social research methods series, vol 23. Sage, Newbury Park, CA
McCorkle R, Quint-Benoliel J (1983) Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med 17:431–438
McCorkle R, Young K (1978) Development of symptom distress scale. Cancer Nurs 1:373–378
Okuyama T, Tanaka K, Akechi T, et al (2001) Fatigue in ambulatory patients with advanced lung cancer: prevalence, correlated factors, and screening. J Pain Symptom Manage 22:554–564
Piper B (1999) Improving the clinical measurement of cancer treatment-related fatigue (abstract). In:Better Health Through Nursing Research: State of the Science Congress Proceedings. American Nurses Association, Washington, DC, p 99
Piper BF, Dibble SL, Dodd M, et al (1998) The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum 25:677–684
Richardson A, Ream E, Wilson-Barnett J (1998) Fatigue in patients receiving chemotherapy: patterns of change. Cancer Nurs 21:17–30
Schwartz AL (2000) Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract 8:16–24
Schwartz AL, Nail LM, Chen S, et al (2000) Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest 18:11–19
So WK, Dodgson J, Tai JSM (2003) Fatigue and quality of life among Chinese patients with hematologic malignancy after bone marrow transplantation. Cancer Nurs 26:211– 219
Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC (1998) Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum 25:915–920
Yamada T, Makita F, Takehara K, et al (1994) Evaluation of the therapeutic effect of TAE on primary liver cancer. Cancer Chemother Pharmacol [Suppl] 33:S55–59
Zeger SL, Liang K, Albert PS (1988) Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049–1060
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Acknowledgement
This study was partly supported by a grant from the National Science Council in Taiwan. The authors gratefully acknowledge the support and assistance of the patients who participated in this study. The authors also thank Claire Baldwin for her English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shun, SC., Lai, YH., Jing, TT. et al. Fatigue patterns and correlates in male liver cancer patients receiving transcatheter hepatic arterial chemoembolization. Support Care Cancer 13, 311–317 (2005). https://doi.org/10.1007/s00520-004-0740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0740-0