Abstract
Goals of work
Prospective clinical study to evaluate patients suffering from solid tumor using a totally implanted venous access device (TIVAD) to determine: (1) if there is a relationship between cutaneous contamination at port insertion site and catheter-related bloodstream infection (CRBI); (2) development modalities of CRBI; (3) if there is a relationship between chemotherapy administration modalities by push/bolus versus continuous infusion and CRBI.
Patients and methods
We studied 41 consecutive patients who needed a TIVAD positioned for chemotherapy administration by bolus/push or continuous infusion. In every patient, we performed blood cultures from blood samples from port catheters and cutaneous cultures from cutaneous tampons of the skin surrounding the implant area on the first (T0) and eight day (T1) postoperatively, after 1 month (T2), and after 3 months (T3) from insertion.
Main results
The study was completed on 40 patients; in one case, the port was removed at T2 for septic complications. We obtained four positive blood cultures (two, 5%), two in the same patient, all caused by staphylococcus. Positive cutaneous tampons were 21 (13%) in 11 patients (27%); the four CRBI occurred in this group of patients with none in the remaining 30 patients (73%) for a total number of 120 tampons (p<0.01). In two cases, the same germ was isolated from both the skin and blood. None of the patients presented a local infection of the subcutaneous pocket. Positive cutaneous cultures decrease over time: T0–T2, 24–5%; T1–T3, 20–5% (p<0.04). There were no differences in CRBI incidence and positive cutaneous tampons between the two chemotherapy administration modalities.
Conclusions
Cutaneous microbial flora has a primary role in CRBI development within TIVADs; there is a relationship between cutaneous colonization and CRBI; colonization reaches its maximum during the first days after catheterization in which the use of the system is at high risk; colonization occurs both via extraluminal and endoluminal routes; there is no difference in CRBI incidence between bolus and continuous infusion administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Totally implanted venous access devices (TIVAD) allow easy and effective treatment for chemotherapy and pain control in cancer patients [1]. However, local and systemic infections still remain the major cause of morbidity and catheter failure [2, 3]. The patient’s cutaneous microbial flora has a fundamental role in the etiology of catheter-related bloodstream infection (CRBI) [4, 5]. These germs initially colonize the external catheter insertion site and later migrate along the catheter’s external surface until reaching the blood (extraluminal route) [6, 7], or they may be directly inserted in the catheter lumen (endoluminal route) [8, 9]. More rare is the contamination of the catheter via the bloodstream from bacteria that originate from a distant focus or the contamination of the fluids or solutions [10, 11].
The highest incidence of extraluminal CRBI occurs during the first week of catheterization, while the endoluminal CRBI occur, generally, later and are associated with the manipulation of the system and the modalities of its use [12].
Compared to indwelling catheters, TIVAD present a smaller incidence of infective complications regardless of the patient’s disease or immunocompetence status and the aggressiveness of the chemotherapeutic treatment [13, 14]. This may depend on the fact that TIVADs are completely imbedded in the subcutaneous layer and, unlike indwelling catheters, do not create continuity between the catheter and patient’s cutaneous microbial flora.
The aim of this study was to evaluate: (1) if there is any relationship between cutaneous bacterial colonization over the skin surrounding the port and development of CRBI; (2) how this develops; (3) if there is any relationship between administration modalities of chemotherapeutic treatment (push versus continuous infusion) and CRBI development.
Patients and methods
We performed a 5-month prospective study on 41 consecutive patients suffering from solid cancer who needed placement of a TIVAD for chemotherapy administration. Each patient was followed for 3 months, from the placement of the device until the last survey. The TIVAD we used were Port-a-Cath (Deltec, USA).
All patients were ambulatory and the surgical procedure, done under strict asepsis, took place in an operating room at a day hospital. The same physician inserted all TIVADs using local anesthetic for a field block and under radioscopic visualization. The catheters were inserted in a central vein using a J guide wire and a peel-away introducing system. At end of the surgical procedure, we flushed the system with 500 U of heparin in 5 ml of solution (100 U/ml) and applied a sterile dressing. In all patients, the system was not used until cutaneous suture removal on the eighth postoperative day.
Chemotherapy administration
According to the different cancer subtypes, patients were treated with two different protocols of chemotherapy administration. All patients were ambulatory; some received repeated bolus administration, others a continuous infusion administered at home by external pump. Patients’ characteristics were evenly distributed between the two groups. For intermittent administration, the port access was carried out with a Huber needle, which was removed after every treatment. In the continuous infusion group, the duration of administration was variable accordingly with the chemotherapeutic protocol in use: 2 days for the de Gramont [15], 5 days in the chronomodulated therapy of colorectal cancer [16], and protracted infusion with low doses of fluorouracil and cisplatin for head and neck cancer and in some other tumor subtypes [17]. In these patients, the port was accessed with a Huber needle, which was removed at the end of every infusion cycle with a maximum duration of 14 days for protracted infusions. In all cases, prior to Huber needle insertion, the skin was disinfected with 10% povidone-iodine solution and, after needle insertion, covered with a sterile and semipermeable dressing.
Microbiology methods
Blood cultures were carried out with the ISOLATOR 10 OXOID method [18]. The ISOLATOR test tube contained some leukocyte and erythrocyte lytic factors. Factors we used were: purified saponin, polypropylene glycol and sodium polyanethol sulfonate. A blood sample (~10 ml) was inoculated in the ISOLATOR 10 test tubes and centrifuged at 3,000 rpm for 30 min. The supernatant was then removed and the pellets seeded on the following culture media: blood agar (anaerobe 35–73°C); chocolate agar (5–10% CO2 35–37°C); Sabouraud’s agar (anaerobe 22–30°C). We checked for bacterial growth every 24 h and eliminated plates at 4, 6, and 8 days respectively.
After incubation, we proceeded with isolation and identification of microorganisms with the VITEK 32 BIOMERIEUX automatic system, and bacteria count was determined with the following formula:
Cutaneous cultures were obtained by passing a swab over the skin surrounding the port, and the cutaneous swabs were then seeded with the “three zone” technique on CAN blood agar, mannitol, MacConkey agar, Sabouraud’s agar, and chocolate agar and incubated for 48 h at 35–37°C. The automatic system VITEK 32 BIOMERIEUX was used for identification of microorganisms.
We considered CRBI positive when cultures of blood samples aspirated from the port developed a count of 100 CFUs or more in absence of any clinical sign related to an infection related to a different focus rather than the central venous catheter (CVC).
Data collection
For every patient, we recorded age, diagnosis, platelet and leukocyte count at time of the surgical procedure, the date of catheter insertion, removal due to any complication, insertion site, use of catheter (intermittent chemotherapy or continuous infusion by external pump), eventual local infections at the site of insertion, results of cultures of blood samples obtained from the TIVAD, and cutaneous cultures from the skin surrounding the device’s area. Both blood and cutaneous cultures were carried out on the first day postoperatively (T0), the eighth day (after cutaneous suture removal and the beginning of chemotherapeutic treatment) (T1), after 1 month (T2), and after 3 months (T3) from implant.
Statistics and data analysis
Different percentages of positive cultures among recorded factors were evaluated with the Fisher exact test.
Results
During the study period, we evaluated 41 implanted TIVADs on 41 patients. The study was completed on 40 patients since in one patient, the device was removed at T2 because of septic consequences. In total, we carried out 163 blood cultures and 163 cutaneous cultures. During catheter placement, none of the patients were affected by fever and/or granulocytopenia.
In 20 patients, the catheter was placed in the right subclavian vein, in 20 in the left subclavian vein, and in one in the right internal jugular. In 22 patients, chemotherapy was carried out by bolus; in the other 19, it was carried out by continuous infusion. Three patients developed four positive blood cultures, which represented 2.5% out of the 163 samples analyzed. In all cases, detected germs were Staphylococcus simulans, capitis, and aureus. S. aureus was isolated at T1 in one patient after he had received antibiotic treatment (teicoplanin 400 mg×2 IV). Afterward, the patient developed septicemia associated with the use of the device, and the same bacterium was isolated from a blood culture at T2. The port was then removed (Table 1), and culture of the catheter tip revealed the presence of S. aureus with the same antibiotic sensitivity test of the germ detected in the blood culture.
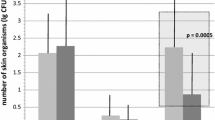
Comparing patients receiving intermittent versus continuous infusion chemotherapy, blood cultures were positive at T1 and T2 in 4.5 versus 0% (NS) and in 4.8versus 5.3% at T3 (NS). Considering both groups of patients, blood cultures appeared to be positive for 2.5% at T1 and T2 and for 5% at T3. Positive cutaneous swabs were 21 (13%) out of 163 on 11 patients (27%); the four positive blood cultures were in this group of patients. The other 30 patients (73%) with 120 negative cutaneous swabs (p<0.01) showed no positive blood culture. No patient, either with positive or negative cutaneous swabs, reported local infection in the subcutaneous pocket where the device had been placed. In positive skin cultures, we detected Staphylococcus in almost all cases, in particular S. aureus, S. epidermidis, and S. simulans; in two out of three patients with CRBI, we detected the same germ at the same time both from skin and blood samples obtained via the TIVAD (Table 1). In positive cutaneous cultures, the difference between continuous infusion chemotherapy and push/bolus chemotherapy reached its maximum value at T1 with respectively 26.3% and 13.6% (NS). The number of positive cutaneous cultures, considering both groups of patients, decreased in time from 24% to 5% (p<0.01) T0- T2 and from 20% to 5% (p<0.04) T1–T3 (Fig. 1).
Discussion
The highest incidence of extraluminal CRBI occurs during the first week after placement of the CVC [12]. An early contamination can occur when viable microorganisms are impacted, during insertion, onto the distal tip of the CVC. Cutaneous microorganisms were detected from skin and from catheter tip samples 90 min after insertion despite rigorous skin disinfection and a strict aseptic technique [19]. An early CRBI can also occur during the days immediately following due to bacterial contamination and growth in the subcutaneous pocket and, subsequently, migration of bacteria along the catheter’s external surface to the bloodstream [6]. Even though TIVADs are completely inserted in the subcutaneous layer, this second mechanism may occur due to the colonization of skin microorganisms that may move by capillary action via the surgical incision into the pocket and then along the dermal tunnel surrounding the catheter. With indwelling catheters, it has been widely proven that cutaneous colonization is strongly predictive for CRBI [20, 21], and also, cutaneous microorganisms surrounding the insertion site are strongly related to germs detected on catheter tip cultures [22, 23].
Given the evidence of the importance of cutaneous microorganisms in the pathogenesis of CRBI, we decided to evaluate if there is any relationship between cutaneous bacterial colonization of the device’s insertion area and CRBI and how this develops. Our results highlight all CRBI in patients with positive cutaneous cultures, and no detectable infection occurred in the group of patients with negative cutaneous cultures. Therefore, TIVADs, as indwelling catheters, present a strong correlation between bacterial colonization surrounding the device’s insertion site and CRBI. This evidence is furthermore confirmed by the fact that in two CRBIs, we detected the same germ at same times in cutaneous tampons and in one case on the catheter tip as well.
Relating to the time of detection, we presume the catheter colonization modalities by cutaneous microbial flora are different in the CRBIs we observed. In early CRBI, observed at T1, when the device had not yet been used and therefore no percutaneous communication with the port had occurred, except for the flushing of the device with heparin after insertion, the catheter colonization took place via extraluminal route probably due to the colonization of the cutaneous wound site of implant and migration to the pocket and along the catheter. We detected the same germ at T1 from both skin surrounding the device’s insertion wound and blood samples obtained via the TIVAD. This was reversed in the two CRBIs that developed at T3 and therefore lengthy use, and the catheter colonization appeared to be via the endoluminal route. The cutaneous microorganisms could have been introduced during the in-use manipulation of the IV line, with the needle during percutaneous puncture of the port, or, in protracted infusions, germs may have colonized the percutaneous needle implanted in the port and successively the catheter. These pathogenic mechanisms can also be responsible for endoluminal CRBIs in TIVADs.
We found no difference in the incidence of CRBI between administration by push/bolus, where manipulation of the system and percutaneous punctures for access to the port are numerous, and the administration by continuous infusion in which the longer transdermal duration of the needle is likely to facilitate germ migration into the port. Similar results have been reported in another study where comparison between chemotherapy administration by push/bolus or by continuous infusion does not highlight any statistically significant difference in the incidence of infections or other complications [24].
We observed colonization of the area surrounding the device’s insertion wound in 11 patients (27%) without any of these presenting a superficial infection of the pocket. Bacterial colonization of a cutaneous, noninfected surgical wound is a relatively common event. In a large study of women undergoing cesarean section, colonization of noninfected wounds occurred in 17% of cases [25] and, in another study of patients undergoing cardiac surgery, colonization of the wound until 6 days post operatively appeared to be related to the antibiotic prophylaxis in use and occurred in 52–78% of cases [26].
It has been demonstrated that there is a wide bacteria colonization of sutures of noninfected wounds, mostly staphylococcus coagulase-negative, which tends to develop in adhesive glycocalix that protects it from the host’s defense mechanisms until suture removal [27]. Microbial colonization of the cutaneous area surrounding the implant’s wound, observed in our study, presents the highest incidence until suture removal (T2) and then significantly decreases with time. This appears to be clinically significant because the density of microbial cutaneous flora is one of the major risk factors for CRBI development, and the system should not be used immediately after the implant. In our institute, we have therefore recommended, as a guideline of good clinical practice, that TIVAD use should follow suture removal from the cutaneous pocket.
In conclusion, our study results have demonstrated: (a) the host’s cutaneous microbial flora, staphylococcus coagulase-negative and S. aureus in particular, have a primary role in CRBI etiology of TIVADs; (b) bacterial cutaneous colonization surrounding the device’s implant area and CRBI development are related; (c) cutaneous colonization occurs in more than one fourth of patients without necessarily presenting a superficial infection of the pocket; (d) cutaneous colonization reaches its maximum, as does the risk of infection, during the days immediately following catheterization and decreases in time; (e) pathogenic mechanisms of catheter colonization for TIVADs, as for indwelling catheters, are the endoluminal route prevalent in CRBIs that arise later and are due to in-use of the IV system; (f) the endoluminal route is more frequent than the extraluminal route (two patients versus one). In the end, we confirmed that there is no difference in CRBI incidence between chemotherapy administrations in bolus versus continuous infusion.
References
Welling RE, Hall JM, Meyer RL Arbaugh JJ(1986) Implantable venous access device: an alternative method of extended cancer care. J Surg Oncol 33:73–75
Grannan KJ, Taylor PH (1990) Early and late complication of totally implanted venous access device.J Surg Oncol 44:52–54
Laurenzi L, Fimiani C, Faglieri N, Natoli S, Milasi G, Tirelli W, Arcuri E (1996) Complications with fully implantable venous access systems in oncologic patients. Tumori 82:232–236
Nouwen JL, van Belkum A, de Marie S, Sluijs J, Wielenga JJ, Kluytmans JA, Verbrugh HA (1998) Clonal expansion of Staphylococcus epidermidis strains causing Hickman catheter-related infections in a hemoto-oncologic departement. J Clin Microbiol 36:2696–2702
Maki DG (1992) Infections due to infusion therapy. In: Bennet JV, Brachman PS (eds) Hospital infections. Little, Brown, Boston, pp 849–898
Mermel LA, McCormik RD, Sprinman SR, Maki DG (1991) The pathogenesis and epidemiology of catheter-related infections with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med 91 [Ssuppl]:S197–S205
Cooper GL, Schiller AL,Hopkins CC (1988) Possible role of capillary action in the pathogenesis of experimental catheter-associated dermal tunnel infections. J Clin Microbiol 26:8–12
Sitges-Serra A,Linares J,Garau J (1985) Catheter sepsis: the clue is the hub. Surgery 97:355–357
Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP (1993) Ultrastuctural analysis of indwelling vascular catheter: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 168:40–47
Maki DG, Jarrett F, Sarafin HW(1977) A semiquantitative culture method for identification of catheter-related infection in the burn patient. J Surg Res 22:513–520
Maki DG, Hassemer CA (1981) Endemic rate of fluid contamination and related septicemia in arterial pressure monitoring. Am J Med 70:733–738
Sitges-Serra A (1999) Strategies for prevention of catheter-related bloodstream infections. Supp Care Cancer 7:391–395
Greene FL, Moore W, Strickland G, McFarland J (1988) Comparison of a totally implantable access device for chemotherapy (Port-A Cath) and long-term percutaneous catheterisation (Broviac).South Med J 81:581–583
Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, Armstrong D (1993) Infectious morbidity associated with log-term use of venous access devices in patients with cancer. Ann Intern Med 119:1168–1174
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer.J Clin Oncol 18:2938–2947
Garufi C, Levi F, Giunta S, Aschelter A, Pace R, Nistico C, Terzoli E (1995) Chronomodulated 5-day infusion of floxuridine and L-folinic acid in patients with advanced malignancies: a feasibility and tolerability study. J Infus Chemother. 5(3) [Suppl 1]:134–137
Arcangeli G, Saracino B, Danesi DT, De Campora E, Giovinazzo G, Cognetti F, Carlini P, Arcangeli S, Mecozzi A (2002) Accelerated hyperfractionated radiotherapy and concurrent protracted venous infusion chemotherapy in locally advanced head and neck cancer. Am J Clin Oncol 25:431–437
Dorn GL, Smith K (1978) New centrifugation blood culture device. J Clin Microbiol 7(1):52–54
Elliott TS, Moss HA, Tebbs SE, Wilson IC, Bonser RS, Graham TR, Burke LP, Faroqui MH (1997) Novel approach to investigate a source of microbial contamination of central venous catheters. Eur J Clin Microbiol Infect Dis 16(3):210–213
Snydman DR, Gorbea HF, Pober BR, Majka JA, Murray SA, Perry LK (1982) Predictive value of surveillance skin cultures in total-parenteral-nutrition-related infection. Lancet 2:1385–1388
Raad II, Baba M, Bodey GP (1995) Diagnosis of catheter-related infections: the role of surveillance and target quantitative skin cultures. Clin Infect Dis 20:593–597
Bozzetti F (1985) Central venous catheter sepsis. Surg Gynecol Obstet 161:593–603
Andremont A, Paulet R, Nitember G, Hill C (1988) Value of semiquantitative cultures of blood drawn through catheter hubs for estimating the risk of catheter tip colonization in cancer patients. J Clin Microbiol 26:2297–2299
Brown DF, Muirhead MJ, Travis PM, Vire SR, Weller J, Hauer-Jensen M (1997) Mode of chemotherapy does not affect complications with the implantable venous access device. Cancer 80:966–972
Martens MG, Kolrud BL, Faro S, Maccato M, Hammill H (1995) Development of wound infection or separation after cesarean delivery. Prospective evaluation of 2,431 cases. J Reprod Med 40:171–175
Palmer DL, Pett SB, Akl BF (1995) Bacterial wound colonization after broad-spectrum versus narrow-spectrum antibiotics. Ann Thorac Surg 59:626–631
Gristina AG, Price JL, Hobgood CD, Webb LX, Costerton JW (1985) Bacterial colonization of percutaneous sutures. Surgery 98:12–19
Acknowledgements
Partially supported by the Italian National Institute of Health, research project N° 0201R2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laurenzi, L., Natoli, S., Benedetti, C. et al. Cutaneous bacterial colonization, modalities of chemotherapeutic infusion, and catheter-related bloodstream infection in totally implanted venous access devices. Support Care Cancer 12, 805–809 (2004). https://doi.org/10.1007/s00520-004-0607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0607-4