Abstract
Insomnia is a common phenomenon in cancer patients; nevertheless, there are only a few intervention results published covering this topic. We examined the effects of a multi-modal psychological sleep management programme combining relaxation techniques, sleep hygiene, cognitive techniques and advice in stimulus control technique on various sleep and quality-of-life variables. We compared two intervention groups up to 6 months after treatment, one with progressive muscle relaxation (n=80), the other with autogenic training (n=71). A control group (n=78) received only a standard rehabilitation programme. It was a heterogeneous sample of adult patients (mean age 58 years) predominantly with breast, kidney or prostate cancer staying for 3 or 4 weeks in an oncological rehabilitation clinic. In comparison to the control group, the analysis of variance for repeated measures (R-MANOVA) showed significant improvements over time, indicating that intervention group participants benefited with moderate- or large-scale effects on sleep latency (p<0.001), sleep duration (p<0.001), sleep efficiency (p<0.001), sleep quality (p<0.001), sleep medication (p<0.05) and daytime dysfunction (p<0.05). In quality-of-life subscales, there was mainly improvement over time. This may indicate a benefit of the rehabilitation treatment in general. No evidence was found for any differences between the two intervention groups. The results suggest that the use of a multi-modal psychological sleep intervention could enhance various sleep parameters and well being of patients. The efficacy on quality of life is still under review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia is reported to be a typical problem of cancer patients, and studies show a higher frequency than in the general population. Although the number of studies evaluating symptoms and causes of insomnia in cancer patients has increased during the last few years, treatment seems to be a neglected problem. Besides hypnotic medication, little has been investigated that might be helpful in improving sleep quality. Therefore the aim of our study was to evaluate the efficacy of standardised multi-modal sleep management training and its impact on quality of life.
Insomnia in cancer patients
The prevalence of insomnia and sleep disturbance in cancer patients has been reported [24, 31, 36, 47, 45] to be much higher (23–61%) than in control groups (about 15%) and the general population (9–30%) [23, 25, 51]. Most studies on cancer patients included heterogeneous samples of newly diagnosed or recently treated patients. Savard and Morin [45] found rates of insomnia between 23% and 44% 2–5 years after treatment, suggesting that insomnia develops a chronic course in a considerable number of cancer patients if untreated [14, 35]. The wide range of results may be based on various factors such as different definitions and diverse methods and instruments in evaluating the extent of insomnia. Also the type of cancer is correlated with the frequency of sleep disturbance: Higher rates were observed in lung and breast than in gastrointestinal, genitourinary and non-melanoma skin cancer patients [15].
The reported types of sleep disorders [15, 22, 24, 31, 36, 48] were characterised by multiple awakenings during the night (from 76% to 90%), sleeping fewer hours than normal (from 84% to 85%), trouble getting back to sleep (from 35% to 75%), trouble falling asleep (sleep latency) (44%) and day-time dysfunction (37%). Davidson et al. [15] found that 59% of their patients had a combination of these problems. Even if sleep disturbance existed before the cancer diagnosis, the symptoms are caused or aggravated by cancer in 58% of cases [45].
Sleep disturbance and possible effects
Several studies showed evidence of links between sleep and psychological or physiological malfunction. Fatigue is the most obvious effect, together with sleeping problems such as insomnia. Though there is much evidence of a correlation between them [10, 13, 16, 29, 33, 39, 49], the mechanism is still not understood completely. Ancoli-Israel et al. [3] postulated in their review that cancer-related fatigue is linked to sleep/wake cycles or to quality and quantity of sleep obtained at night. Further effects of sleep problems on mood disturbances or psychiatric disorders were discussed.
Physiologically different kinds of health problems and physical symptoms (e.g., headache, stomach discomfort and pain) were discussed [30] and particularly immunosuppressive effects. Some studies showed a positive correlation between sleep disturbances in general and an immune deterioration [18, 27, 28]. In our study, we often noted anxiety in our patients when reporting their sleep problems.
Treatment of sleeping problems in cancer patients
Most cancer patients did not ask their physicians for help. Only 16.6% of the patients commented on sleep disturbances [22]. Engstrom et al. [22] found that most patients seek support by sharing their problems with others (50%) or by using other strategies such as reading or taking medication (35%). Patients who asked their physicians for help were commonly prescribed psychotropic medication [17]. Savard and Morin [45] pointed out the side effects of most prescribed drugs such as benzodiazepines and concluded that a 4-week restriction was necessary to minimise the risk of tolerance or dependence.
There are a number of efficient psychological intervention techniques available to reduce sleep disturbance in patients with primary insomnia. Savard and Morin [45] reported in their review on moderate-to-large effect sizes on sleep onset latency, sleep quality and duration of awakenings. Most of the examined study designs used behavioural treatments (e.g., stimulus control, sleep restriction, sleep hygiene) and/or cognitive treatment (e.g., relaxation techniques, cognitive therapy).
With regard specifically to cancer, there are only a few studies that investigated the efficiency of psychological treatment [11, 19, 50, 26, 46, 44, 52]. Stam and Bultz [50] demonstrated good results with a short-term (five-session) progressive muscle relaxation (PMR) and autogenic training (AT) with a cancer patient on his sleep latency (from 1.9 to 0.7 h) and sleep duration (from 4.4 to 7.1 h). Cannici et al. [11] carried out a muscle relaxation training programme that was administered in individual sessions on three consecutive days. They designed an intervention group receiving the training and a control group with routine care. The sleep onset of the intervention group was reduced significantly from 124 to 29 min while the control group remained largely unchanged with a reduction from 116 to 109 min. Wright et al. [52] used autogenic training in a heterogeneous group of cancer patients. The qualitative evaluations of the group illustrated improved sleep. Dolan [19] found that a combined programme of muscle relaxation, thought stopping, focused attention and positive coping statements worked effectively on reducing sleep disturbances in seven cancer patients.
In recent studies by Savard et al. [46] and Quesnel et al. [44] using a multi-modal cognitive-behavioral treatment combining stimulus control, sleep restriction, cognitive therapy and sleep hygiene education on breast cancer patients, the authors reported significant improvements in sleep efficiency and total wake time. These results were confirmed by polysomnographic outcomes. The effects were associated with improvements in mood, reduced fatigue and global and cognitive quality of life dimensions.
Sleep disturbance and quality of life of cancer patients
Quality of life of cancer patients is a frequently evaluated topic [9, 21, 43]. A recently published study showed that following treatment, an improvement in sleep parameters correlated significantly with higher scores in global and cognitive quality-of-life dimensions [44].
Patients and methods
Design and procedures
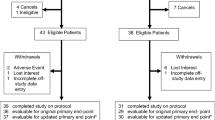
This study used four time points of measurement (beginning and end of rehabilitation, 6 weeks and 6 months later) and cross-sectional examination of cancer patients with two intervention groups and one control group. Adult cancer patients were recruited in a cancer rehabilitation clinic where they stayed for 3 or 4 weeks after having received acute surgery, chemotherapy or radiation treatment. The study took place for 3 years. Patients with sleep disturbances were asked by their physicians to participate. Participation was entirely voluntary; sleep intervention was given in addition to the standard rehabilitation treatment, so no one would be disadvantaged if not participating. Due to the cancer-related handicaps, the standard treatment included specific physical training to reduce handicaps, medical and dietary counselling, relaxation training (two-to-three times a week), psychosocial groups to activate psychic resources and to support a healthy lifestyle and other individually selected components.
The control group patients were informed that we wanted to investigate their sleep behaviour. This group was recruited some months prior to introducing the intervention programme and during a longer therapists’ holiday break some months later. By picking these time periods, we made sure that we had no biases in the control group due to time of year. Control group patients were given the above-mentioned standard medical and psychosocial treatment.
The physicians checked at the beginning that the diagnostic criteria for insomnia (DSM-IV) [2] were satisfied. They filled in a medical information form created for the study. Personal data was gathered by a short questionnaire. The third and fourth consultations were done by post. The study was approved by an ethical commission.
Intervention programme
The two intervention groups proceeded with two different relaxation techniques: PMR or AT. Patients could decide for themselves at the beginning which relaxation technique they wanted to learn. First they were offered a test session in each technique as an additional help for their decision and decided afterwards which technique was more comfortable to them. This approach corresponds to our experience that the participants profit more if the relaxation technique is suitable to them, and it fits to the ethical guidelines of our clinical concept to give them the optimum treatment possible. If a patient had no preference, he or she was placed by turns into the AT and PMR group.
Both groups received three standardised psycho-educational sessions 1 h each providing information about sleep, faulty beliefs about sleep disturbance, relaxation techniques, sleep hygiene, stimulus control technique similar to Bootzin et al. [7] and cognitive techniques like thought stop and guided imagery. Each session started with a short conversation concentrated on intervention techniques and sleep in general designed to correct false expectations.
The relaxation techniques were modified to support falling asleep [5]. After the first session, the participants were handed cassette players with cassettes containing their relaxation technique and instructions to use it regularly (daily). The “normal” relaxation technique without any suggestion to fall asleep was learned in a group setting simultaneously as part of the standard rehabilitation treatment in the clinic. Here participants were instructed to stay awake. Participants were also given handouts to work with (e.g., for sleep hygiene). The last session set up individual strategies to transfer the outcomes into the future (relapse prevention and motivation strategies).
Instruments
Personal and medical information
In order to establish a baseline, we created a standardised information form with a section for the physician and another for the patient to evaluate demographic, cancer-related and sleep-related data. The physician also checked whether the patient met the criteria for insomnia. For the third and forth survey, the patients filled in a short questionnaire on life events and use of the above relaxation techniques.
Sleep questionnaire
To our knowledge, there exists no quantified measure of sleep disturbance or insomnia for cancer patients in the German language. Therefore we used a questionnaire derived from the German translation of the Pittsburgh Sleep Quality Index (PSQI) [4].
The PSQI is a standardised questionnaire evaluating different scores with regard to sleep latency, sleep duration, sleep efficiency, sleep quality and sleep disturbance, use of sleep medications and daytime dysfunction [12]. We did not compute generated scale scores, only using raw data. High level scores in the PSQI indicate more discomfort. We added one more scale called “daytime energy” to explore a more positive attitude than daytime dysfunction: “How would you describe your daytime fitness/energy during the day for the last 2 weeks?”
To evaluate quality of life, we used the Cancer Quality of Life Questionnaire 30 of the European Organisation for Research and Treatment (EORTC-QLQ-C30), the most often-used instrument for this purpose [21]. We handed out the PSQI and the EORTC-QLQ-C30 on all four dates.
Data analysis
Statistical analysis was conducted electronically with SPSS (version 10). To examine initial differences in demographic, cancer-related, sleep and quality-of-life characteristics between the three groups (control, PMR, AT) that might interfere as potential covariates, we used ϰ2 tests and conducted ANOVA.
To explore the differences in sleep parameters between the three groups over the four points of time (T0=baseline, T1=end of rehabilitation 3 or 4 weeks later, T2=6 weeks after T1, T3=6 months after T1), we conducted an analysis of variance for repeated measures (R-MANOVA) in which the sub-scales of the PSQI served as dependent variables and groups and points of time served as independent variables.
To examine differences in quality of life between the three groups over the four points of time, we performed an R-MANOVA in which the scales of the EORTC-QLQ-C30 served as dependent and groups and points of time served as independent variables.
Results
Demographic sample characteristics
Demographic and cancer-related characteristics of the different groups are listed in Table 1. The mean age in the control group (C group) was 57.6 (SD=10.9, n=78), in the PMR group, 60.2 (SD=9.2, n=80) and in the AT group, 57.6 (SD=11.7, n=71). The mean duration of insomnia of all three groups was 101.3 months. Each group consisted mainly of women; the typical participant was married, retired or employed, tended to feel in a financially safe situation, had a cancer severity index of 1 (WHO index), had undergone two different types of medical treatment (surgery, chemotherapy, radiotherapy or anti-hormone therapy) before rehabilitation and reported mild or rare pain. The most frequent type of cancer was breast, followed by kidney and prostate. The mean pain status on a five-point scale (0=no pain up to 5=severe pain) was 1.1 in the control group (SD=1.0), 1.0 in the PMR group (SD=1.3) and 0.9 in the AT group (SD=1.2). All participants were Caucasian and almost all of German origin. None of these demographic, sleep-related or cancer-related variables differed significantly between the groups.
The mean duration of insomnia or sleep disturbance varied between 91 and 116 months implying that most cancer patients experienced sleeping problems long before the diagnosis of cancer was given, which ranged in our sample from a few weeks up to 2 years.
Sleep parameters
Table 2 shows the results of the multi-variate tests performed on each of the sleep subscales. Examination of the baseline data revealed no significant differences between the three groups, although we found trends in sleep latency and sleep duration indicating that the participants in the control group tended to experience shorter sleep latency and longer sleep duration. Sleep latency and duration are described in minutes and sleep efficiency in percent.
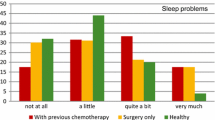
In the following scales higher scores represent greater problems. Sleep disturbance is composed of 11 items like “too hot”, “too cold” or “using the bathroom” and ranges from 0 to 33. Daytime energy, sleep medication and sleep quality consist of only one question each, ranging between 0 and 3; daytime dysfunction is composed of two questions and ranges from 0 to 6.
A comparison of the first and the last point of time for each subscale gives the following results: Sleep latency (T0–T3) improved significantly over time and group (F=8.6; p<0.001). Patients in the intervention groups profited considerably more from the training than the control group, yet there was a significant improvement over time for the control group as well. The greatest effect could be seen within the first 3 or 4 weeks (T0–T1).
Sleep duration (T0–T3) increased significantly over time and group (F=10.1; p0<.001) as well as sleep efficiency (F=10.5; p<0.001). The main effect could be found between T0 and T1. Afterwards both improved slightly over time. In sleep disturbance, the results showed a significant improvement over time for all groups (T0–T3; F=57; p<0.001). Analysing the group differences, only the AT group profited significantly (T0–T3; F=6.6; p=0.01). The general time effect was higher than the group effect. Sleep quality improved significantly over time (T0–T3; F=232; p<0.001) over the entire study. Altogether, daytime energy improved significantly over time (T0–T3; F=114; p<0.001) as did daytime dysfunction over time and group (T0–T3; F=3.5; p<0.05). Nevertheless, in both we found a deterioration from T1 to T2 with only a marginal change to T3. Sleep medication decreased significantly (T0–T3; F=4.1; p<0.05) over time and group from T0 to T1 and remained up to the end.
We found no negative effects on the participants associated with the intervention improvements. The effect sizes of the positive effects (over time or over time and group) ranged between 0.5 and 1 standard deviation.
Comparison between PMR and AT groups
To examine possible differences in the effects of PMR and AT on sleep parameters, we conducted a multi-variate analysis (R-MANOVA). There were no significant differences between the groups, indicating that both techniques worked equally well.
Quality of life
Table 3 shows the results of multi-variate tests performed on the EORTC-QLQ-C30 sub-scales. We chose all functional scales for this study, excluding scales in this article less relevant to sleep (nausea and vomiting, dyspnoea, appetite loss, constipation and diarrhoea). Examination of the baseline data (T0) revealed no significant differences or tendencies between the three groups. A comparison of the first and last point of time for each sub-scale gave the following results: With the exception of pain, which started on a low level and did not differ significantly, patients of all groups improved in all scales comparing T0 with T3 (physical functioning: F=10.4, p<0.001; role functioning: F=16.2, p<0.001; emotional functioning: F=52.0, p<0.001; cognitive functioning: F=14.1, p<0.001; social functioning: F=24.1, p<0.001; global quality of life: F=34.0, p<0.001; fatigue: F=76.8, p<0.001; sleep disturbance: F=209, p<0.001; financial impact: F=5.2, p<0.005). In the global quality-of-life scale, the result proved to be significantly different over group as well (F=3.6, p<0.05): The PMR and AT participants benefited more than the control group members. The result in sleep disturbances showed the same significant effect (F=4.8, p=0.03), but only for the AT group. The control group did not differ from the PMR group.
The effect sizes of the quality-of-life sub-scales (T0–T3) proved to be low in the physical, role and cognitive functioning and financial impact scales. They were moderate in the emotional and social functioning, global quality of life and fatigue scales. They were high in the sleep disturbance scale.
Referring to time effects we found distinct improvement mainly at the end of the rehabilitation (T1) then later on, slight deterioration in most scales during 6 weeks after the treatment (T2), which remained more or less fixed at the last point of time (T3). Sleep disturbance was the only exception in this process improving from the beginning up to the end continually. Despite the disadvantages from T1 to T2, overall, patients improved over the 6 months, as shown above.
Discussion
This study was designed to gather information on the effects of a concise multi-modal psychological programme in treating insomnia of cancer patients. The patients stayed for 3 or 4 weeks in a rehabilitation clinic with the intention of reducing disease- or treatment-related handicaps. The insomnia treatment was given in addition to the standard medical and psychosocial treatments.
When we started in 2000, we chose a multi-modal concept to overcome the limitations reported in former publications [11, 50] by using only a single relaxation technique. In both studies, the participants reduced their sleep latency to approximately 30 min and prolonged their sleep duration to approximately 6.5 h. Nevertheless, Cannici et al. [11] stated that there were no significant decreases in nocturnal thoughts or nervousness in their subjects. Stam and Bultz [50] found no changes in the difficulty of their patients in keeping thoughts out of mind. In both studies, sleep quality, efficiency and effects on daily functioning and well being were not included.
Relaxation techniques such as PMR and AT claim to contain both a cognitive distraction and a relaxation component [34, 52]. While the diversion of the patients’ attention from distressing factors works in a different context such as chemotherapy [38] and stress [6] or in the context of insomnia without the cancer problem [4], it seems to be less effective in this context, i.e. cancer patients with insomnia. In our group sessions, patients frequently reported that they felt uncomfortable or unable to practice relaxation techniques when they were distracted by persistent thoughts and/or feelings like anxiety or depression. A more active strategy therefore, like thought stop, special concentration and meditation exercise seems more appropriate in these situations. Opting for a multi-modal concept matches with recent results of Quesnel et al. [44], which found significant improvements in a survey of eight patients in various scales. The positive results on sleep variables were corroborated by polysomnographic evaluation.
The most striking effects over time and group found in our study were those of improvement in sleep latency, sleep duration, sleep efficiency, sleep medication and daytime dysfunction, showing that the intervention groups benefited most. The greatest benefit could be observed within the first 3 or 4 weeks. This appears to be a rapid process, but it corresponds with the results of Cannici et al. [11] (9 days), Stam and Bultz [50] (5 weeks) and Quesnel et al. [44] (8 weeks). Lacks and Morin [32] reviewed the effects of different techniques on insomnia finding that the majority of participants benefited 2 months after the end of the treatment and the improvement remained.
An achieved sleep latency of about 30 min (PMR and AT group) in our own study is usual for people without sleep disturbance. Sleep duration of nearly 6.5 h is lower than average [40], but as Quesnel et al. [44] pointed out, results of patients with primary insomnia benefiting from psychological intervention show that only a few of them become really good sleepers [20]. Given that the duration of sleep varies individually, the increase of sleep efficiency proves to be more significant. Totals of 73% (PMR) or 78% (AT) are normal or indicate only slight disturbance in reference to common standards [40].
Sleep disturbance did not change significantly. This component scale of 11 items should be investigated more closely in a future analysis. We assume that special cancer-related handicaps, like micturition at night or hot flushes due to taking hormone medication, influence the results. Therefore, this component scale has to be differentiated to identify possible reasons.
While the use of sleep medication did not change in the control group, it was halved in the intervention groups. This decrease fits the findings of Quesnel et al. [44] and proves the benefit of this training taking into account the side effects of drug use.
Though daytime dysfunction and daytime energy both show significant improvement over time, there appeared to be a slight deterioration after the rehabilitation. These findings can be seen in most quality-of-life parameters as well. Since we did not establish a control group without any treatment, we are somewhat limited in the interpretation of this process. Nevertheless, we are convinced that this universal improvement in sleep and quality-of-life parameters during rehabilitation is a general effect of the rehabilitation treatment itself. Concerning the quality of life and the daytime-related sleep scales, this effect decreases after the treatment. Probably the daily concerns and worries back home lessen the positive results of the interventions, though data after 6 months are still significantly better than before treatment.
As mentioned before, quality of life improved slightly. Only the global and the sleep disturbance factors gave evidence over time and over group from beginning to end of rehabilitation. It may be that the positive effects of both the standard rehabilitation treatment in the clinic and the sleep management training produce this feeling of well being and the reduction of fatigue symptoms. Despite the findings of Passik et al. [42], who attempted unsuccessfully to develop a single-item screening instrument using one item from the Zung Self-Rating Depression Scale, the QLQ-C30 item seems to be a sensitive screening instrument.
Similar to the findings of Engstrom et al. [22], we experienced the necessity to approach our patients and encourage them to participate in the intervention program. The vast majority was very pleased to be given the opportunity to participate.
In conclusion, the evaluated multi-modal management training for insomnia in cancer patients appears to be efficient. Most of the sleep parameters improved to a moderate or large extent. Patients in the intervention groups benefited more than the control group members. To some extent, all patients benefited from the basic rehabilitation treatment itself with regard to sleep and quality of life.
Future studies should analyse in a more differentiated way the interfering co-variates like hormone therapy and their effects on the various sleep variables. The intervention should probably be more specific to help these patients. Additionally, future investigations should examine who benefits most from the intervention by developing a screening instrument to identify the patients who should or should not attend, and secondly to develop alternatives for those who do not benefit from the intervention method chosen.
References
Aaronson NK, Ahmedzai S, Bergman B (1993) The EORTC QLQ-C30: A quality of life instrument for use in international clinical trials in oncology. J Nat Canc Inst 85:365–376
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington DC
Ancoli-Israel S, Moore PJ, Jones V (2001) The relationship between fatigue and sleep in cancer patients: a review. Europ J Canc Care 10:245–255
Backhaus J, Riemann D (1999) Schlafstörungen. Hogrefe, Göttingen Bern Toronto Seattle
Behre A, Scholz OB (1998) Behandlungswirkungen standardisierter hypnotherapeutischer Suggestionen bei Patienten mit Schlafstörungen. Hypnose und Kognition 15:113–127
Bernstein DA, Borkovec TD (1973) Progressive Relaxation Training. Research Press, Champaign
Bootzin RR, Epstein D, Wood JM (1991) Stimulus control instructions. In: Hauri P (ed) Case studies in insomnia. Plenum, New York, pp 19–28
Borkovec TD, Fowles DC (1973) Controlled investigation of the effects of progressive and hypnotic relaxation on insomnia. J of Abnorm Psychol
Bottomley A, Vanvoorden V, Flechtner H, Therasse P (2003) The challenges and achievements involved in implementing quality of life research in cancer clinical trials. Eur J Cancer 39:275–285
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR (2000) Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol 18:743–753
Cannici J, Malcolm R, Peek LA (1983) Treatment of insomnia in cancer patients using muscle relaxation training. J Behav Ther Exp Psychiatry 14:251–256
Carpenter JS, Andrykowski MA (1998) Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 45:5–13
Cimprich B (1999) Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs 22:185–194
Couzi RJ, Helzlsouer KJ, Fetting JH (1995) Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol 13:2737–2744
Davidson JR, MacLean AW, Brundage MD, Schulze K (2002) Sleep disturbance in cancer patients. Soc Sc Med 54:1309–1321
de Jong N, Courtens AM, Abu-Saad, HH, Schouten, HC (2002) Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Canc Nurs 25:283–299
Derogatis, LR, Feldstein M, Morrow G, et al (1979) A survey of psychotropic drug prescriptions in an oncology population. Cancer 44:1919–1929
Dinges DF, Douglas SD, Zangg L, Campbell DE et al (1994) Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93:1930–1939
Dolan JD (1984) The self-management of sleep interruptions, pain and nausea by adult oncology outpatients: An investigation. Diss Abstr Int B 45:826
Edinger JD, Wohlgemuth WK (1999) The significance and management of persistent primary insomnia: The past, present and future of behavioral insomnia therapies. Sleep Med Rev 3:101–118
Efficace F, Bottomley A, van Andel G (2003) Health related quality of life in prostate carcinoma patients: a systematic review of randomized controlled trials. Cancer 97:377–388
Engstrom C, Strohl R, Rose L et al (1999) Sleep alterations in cancer patients. Canc Nurs 22:143–148
Ford D, Kamerow D (1989) Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA, 262:1479–1484
Fortner, B. Stepanski E, Wang S. et al (2002) Sleep and quality of life in breast cancer patients. J Pain Symptom Manage, 24:471–480
Holzrichter S, Hajak G, Rudolph, G, Westenhöfer F, et al (1994) Wie schlafen die Deutschen–eine Repräsentativumfrage in Westdeutschland. Medizin Wochenschr (Sonderheft) 62–73
Hu D, Silberfarb PM (1991) Management of sleep problems in cancer patients. Oncology 5:23–28
Irwin M, Smith T, Gillin C (1992) Electroencephalographic sleep and natural killer cell activity in depressed patients and control subjects. Psychosom Med 54:10–21
Irwin M, Fortner M, Clark C, McClintick J, Costlow C et al (1995) Reduction of natural killer cell activity in primary insomnia and in major depression. Sleep Res 24:256
Jereczek-Fossa BA, Marsiglia HR, Orecchia R (2002) Radiotherapy-related fatigue. Cirt Rev Oncol Hematol 41:317–325
Kales, JD, Kales A, Bixler EO et al (1984) Biopsychobehavioral correlates of insomnia. V: Clinical characteristics and behavioral correlates. Am J Psychiatry 141:1371–1376
Kaye J, Kaye K, Madow L (1983) Sleep patterns in patients with cancer and patients with cardiac disease. J Psychol 114:107–113
Lacks P, Morin CM (1992) Recent advances in the assessment and treatment of insomnia. J Consult Clin Psychol 60:586–594
Lichstein KL, Means MK, Noe SL, et al (1997) Fatigue and sleep disorders. Behav Res Ther 35;733–740
Linden W, (1990) Autogenic Training: a Clinical Guide. Guilford, New York
Lindley, C, Vasa S, Sawyer WT, Winer EP (1998) Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer J Clin Oncol 16:1380–1387
Malone M, Harris AL, Luscombe DK (1994) Assessment of the impact of cancer on work, recreation, home, management and sleep using a general health status measure. J R Soc Med 87:386–389
Mellinger GD, Balter MB, Uhlenhuth EH (1985) Insomnia and its treatment Prevalence and correlates. Arch Gen Psychiatry 42:225–232
Molassiotis A, Yung HP, Yam BM, Chan FY, Mok TS (2002) The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomised controlled trial. Supp Care Cancer 10:237–246
Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Mattesou S (2002) Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Supp Care Cancer 10:389–398
Müller T, Paterok B (1999) Schlaftraining. Hogrefe. Göttingen
Nicholi AM (1978) The Harvard guide to modern psychiatry. Belknap, Cambridge, MA
Passik SD, Whitcomb LA, Kirsh KL, Theobald DE (2003) An unsuccessful attempt to develop a single-item screen for insomnia in cancer patients. J Pain Symptom Manage 25:284–287
Penson DF, Litwin MS, Aaronson NK (2003) Health related quality of life in men with prostate cancer. J Urol 169:1653–1661
Quesnel C, Savard J, Simard S, Ivers H, Morin CM (2003) Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 71:189–200
Savard J, Morin CM (2001) Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol 19:895–908
Savard J, Simard S, Quesnel C et al (2000) Preliminary results of a randomized trial on the efficacy of cognitive-behavioral treatment for insomnia in breast cancer. Int J Behav Med 7:35
Savard J, Simard S, Blanchet J, Ivers H, Morin CM (2001) Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep 24:583–590
Silberfarb PM, Hauri PJ, Oxman TE, Lash S (1985) Insomnia in cancer patients. Soc Sc Med 20:849–850
Smets EM, Garssen B, Schuster-Uitterhoeve AL, de Haes JC (1993) Fatigue in cancer patients. Br J Cancer 68:220–224
Stam HJ, Bultz BD (1986) The treatment of severe insomnia in a cancer patient. J Behav Ther Exp Psychiatry 17:33–37
Weyerer S, Dilling H (1991) Prevalence and treatment of insomnia in the community: Results from the Upper Bavarian filed study. Sleep 14:392–398
Wright S, Courtney U, Crowther D (2002) A quantitative and qualitative pilot study of the perceived benefits of autogenic training for a group of people with cancer. Europ J Canc Care 11:122–130
Acknowledgements
This study was funded by grant number 50–34 from the Verein zur Förderung der Rehabilitationsforschung in Schleswig-Holstein e.V. (vffr). We also wish to express our appreciation to Jens Bade as project director and Gary Hlavaty, Christian Simeit, Randolph Krebs, Anja Pliske and Andrea März for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simeit, R., Deck, R. & Conta-Marx, B. Sleep management training for cancer patients with insomnia. Support Care Cancer 12, 176–183 (2004). https://doi.org/10.1007/s00520-004-0594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0594-5