Abstract
In recent years a paradigm shift towards a patient-focused rather than a disease-focused approach occurred in many health care systems. The pharmacy profession experienced an accordant development. The traditional drug-oriented services expanded towards patient-oriented services. In oncology, pharmacists established central services for compounding of cytotoxic drugs and offered therapeutic drug monitoring for critical substances. Pharmaceutical care concepts are now being introduced to optimize individual drug therapy. Pharmaceutical care aims at improving safety and therapeutic outcomes and consequently, the patient’s quality of life. These objectives imply a close relationship to supportive care. To achieve this, a multidisciplinary approach seems to be beneficial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to international concerted cancer research, nowadays patients can be offered an individually tailored therapy. Systemic therapies are part of most therapeutic algorithms, and for some malignancies, they even seem to be the only option. More cancers are curable or can be halted in a chronic state, which goes along with changing patient needs. This is why in recent years a paradigm shift occurred towards a patient-focused rather than a disease-focused approach. Patients’ quality of life during and after chemotherapy emerged as an important outcome parameter alongside the tumor response. Therefore, the patient has to be offered an appropriately indicated, effective, safe, and convenient drug therapy. Supportive therapy became an integral part of anticancer therapy to limit therapy-associated toxicity. Moreover, the significance of complementary therapy options for cancer patients became evident. However, not only the anticancer drugs have to be taken into consideration. The patient often has to take additional medication against other underlying conditions such as asthma, diabetes, etc.
The more complex drug regimens get, the higher is the risk of experiencing drug-related problems (DRP). Drug-related problems in cancer chemotherapy can have severe consequences originating from the high toxicity of anticancer drugs. They may arise from lack of adherence to the protocols, or they may be associated with the chemotherapy itself or with inadequately applied supportive medication. Numerous attempts have been made to improve the prevention of medication errors in chemotherapy. Additional to systematic changes, prevention strategies should be applied on the individual basis.
Adverse drug reactions (ADR) represent a particular group among the DRPs. According to the World Health Organization, ADRs are any noxious, unintended, and undesired effects of a drug, which occur at doses used in humans for prophylaxis, diagnosis, or therapy. This definition excludes therapeutic failures, medication errors, and abuse. Lazarou et al. found that fatal ADRs ranked between the fourth and sixth leading cause of death in US hospitals in 1994 [17]. These results only refer to serious and fatal ADRs. This impressively illustrates how crucial the prevention of ADRs and thus DRPs is.
Not only for safety reasons does it seem important to reduce the incidence of ADRs. Studies conducted in US nursing homes have shown that with every dollar spent on drugs US $1.33 have to be afforded for treating adverse drug events [2]. Therefore, the prevention or the detection of drug-related problems at an early stage has also a large potential for cost reduction.
The American Federation of Clinical Oncologic Societies addressed these aspects in their consensus statement on quality cancer care. They ask for multidisciplinary teams of oncologic health care professionals to guarantee optimal treatment outcome for the cancer patient [3]. As a multidisciplinary organization, the Multinational Association of Supportive Care in Cancer (MASCC) aims to promote professional expertise in supportive care through research and international scientific exchange of ideas. With their knowledge about drugs, pharmacists may contribute in different ways to improve cancer care and complement the multidisciplinary cancer care team.
Oncology pharmacy
As with other professions, the pharmacy profession experienced a change from traditional drug-oriented services, such as drug distribution and preparation toward patient-oriented services. Within the last decade, the specialty of oncology pharmacy developed and gained knowledge and experience to serve the expanding demands of the health system regarding cancer care. The setting up of central services for compounding of cytotoxic drugs and standardization of the chemotherapy order forms was one of the first pharmaceutical contributions to decrease prescribing and dosing errors and to increase the safety in handling cytotoxic drugs. Meanwhile the list of oncology pharmacy services expanded considerably, as shown in Table 1. To illustrate the development of oncology pharmacy, some services are described in more detail.
Therapeutic drug monitoring
The generally narrow therapeutic range of anticancer drugs means a particular risk for the patient in terms of drug safety. The relationship between the systemic exposure of cytotoxic drugs and their desired and undesired effects is widely recognized. For drugs such as fluorouracil, mercaptopurine, and methotrexate, a relationship between pharmacokinetics and treatment outcome has been shown. For other anticancer drugs such as platinum complexes, anthracyclines, and some antimetabolites, a relationship between the serum concentrations and the respective dose-limiting toxicity is described [14]. Therapeutic drug monitoring (TDM) aims to optimize individual dosing and hence to maximize efficacy and minimize toxicity. Still, the strategy has its limitations due to a number of reasons. For one thing, the drugs are usually given in combination, and it is difficult to estimate the pharmacodynamic effects of individual agents. Furthermore, the target tissues are often remote from the plasma being the matrix used for analysis, which may confound the interpretation. Hon and Evans addressed this problem and stated that the optimal use of TDM can only be achieved with an effective cooperation of a multidisciplinary team of clinical professionals [14]. Pharmacists not only offer TDM using established methods, e.g., for methotrexate and aminoglycosides, but also work on teams to find new analytical strategies and to make the methodology available for more anticancer drugs.
Elaboration of therapeutic guidelines
Therapeutic guidelines should be elaborated in a multidisciplinary team approach with physicians, pharmacists and other health care professionals. Their consequent implementation can contribute to improve the patients’ quality of life and help reduce unnecessary drug costs. Among others, Dranitsaris et al. showed in a prospective intervention study that the implementation of evidence-based antiemetic guidelines with the support of pharmacists could promote the clinically appropriate use of 5-HT3 antagonists. The therapeutic outcome for the patient could be improved and drug costs could be reduced [8].
Pharmacoeconomics
Due to cost pressure in the health care sector, treatment costs play an increasingly important role in supportive care. Therefore, guidelines have been established that are not only based on randomized clinical trials but also include pharmacoeconomic evaluations. The 5-HT3 antagonists in the treatment of chemotherapy-induced nausea and vomiting are a good example. Whereas for acute emesis 5-HT3 antagonists show a pharmacoeconomic benefit compared to high doses of metoclopramide, they should not be used for the treatment of delayed emesis. A study by Berard et al. showed that the implementation of a treatment algorithm for emesis, incorporating aspects such as treating delayed nausea and vomiting without using 5-HT3 antagonists or adapting antiemetic prophylaxis and treatment to the emetogenic potential of the chemotherapy regimen, led to a cost reduction of about US$205,000 in a 719-bed medical center in the first year [5]. Thus, the right choice of treatment can save a substantial amount of money. Furthermore, the route of administration has a great impact on treatment costs. Frequently, oral administration is as efficient as intravenous administration but at significantly lower costs. Engstrom et al. showed that the implementation of an oral antiemetic regime was able to save about US$20,000 per year [9].
Oncology pharmacy societies
The establishment of the specialty is expressed in the foundation of oncology pharmacy societies on the national and international level. The International Society for Oncology Pharmacy Practitioners (ISOPP) was founded in 1995. The aim of the society is to ensure the optimal medical treatment for cancer patients and thereby improving their quality of life. Among other goals, it aims to set up collaborative relationships, introduce international standards in oncology pharmacy practice, and establish research standards in this field. The society’s print medium is the Journal of Oncology Pharmacy Practice (JOPP), which reflects the ambition to contribute scientific publications to support the developing field of oncology pharmacy.
The German section of the society developed and published the quality standards for the oncology pharmacy service (QuapoS). The standards started off by giving advice on the safe handling of cytotoxic drugs in preparation, distribution, and administration, to protect the preparing pharmacist, the courier, the nurse, and the patient. The technical and personnel requirements for compounding of cytotoxic drugs were defined. In the current third edition, pharmaceutical care and—as a major part of it—supportive care are included as areas for oncology pharmacists to contribute to individual patient care [21]. The QuapoS were translated into English and a number of other languages to contribute to the international discussion. These developments form the structural and theoretical basis for quality oncology pharmacy services.
To assure the implementation to practice, a further educational program has been available to German pharmacists since 2000 to specialize in oncology pharmacy. One hundred seminar hours must be attended to become a specialist for oncology pharmacy. Content extracts are listed in Table 2 [15]. In education programs for pharmacists, for example in the US, the care of cancer patients is also an integrated part [19].
Pharmaceutical care
Definition and philosophy
The recognition of the numerous risks to the individual patient associated with complex drug therapies has led to the development of a conceptual framework for an advanced pharmacy practice philosophy. In 1990, Hepler and Strand introduced the concept of pharmaceutical care as a further development of the pharmaceutical profession [12]. They understand “pharmaceutical care as the responsible provision of drug therapy for the purpose of achieving definite outcomes that improve a patient’ quality of life.”
The Fédération International Pharmaceutique (FIP) extended this definition in 1998, describing it as a “collaborative process that aims to prevent or identify and solve medicinal product and health-related problems. This is a continuous quality improvement process for the use of medicinal products.” Pharmaceutical care is a comprehensive practice model. It should be offered to the patient as a whole.
The American Society of Health System Pharmacists set up guidelines on a standardized method for pharmaceutical care to assure that pharmacists practicing pharmaceutical care work on the same quality level [4]. These guidelines amalgamate the aspects introduced above. The London Oncology Pharmacy Group also introduced guidelines for the pharmaceutical care of the cancer patient that not only include the actual “pharmaceutical care” as such, but also standardizes other pharmaceutical activities including dispensing, updating therapeutic policies, reconstitution of cytotoxic drugs, drug information, contribution to clinical trials, and the oncology training of pharmacists [13].
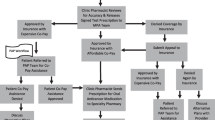
A fundamental development of pharmaceutical care compared to other pharmaceutical services is that pharmacists accept responsibility for the patient’s pharmacotherapeutic outcome alongside the physicians. Consequently, this concept only works in close collaboration with the other involved parties, as shown in Fig. 1.
Pharmaceutical care as a needs-based approach
For pharmaceutical care providers, the main focus is the drug-related needs of the individual patient. Individual drug therapy should be appropriately indicated, effective, safe, and convenient to the patient to assure good compliance and thus optimal treatment outcomes [7]. These drug-related needs are not necessarily met, which can result in a variety of drug-related problems (see Table 3).
The care process
Pharmaceutical care is provided as a continuous process that is structured according to the SOAP method: Subjective information and objective parameters of the individual patient are analyzed and used to design an individual care plan. In collaboration with the prescribing physician and the patient, the goals of the individual drug therapy are to be defined and added to the plan. Regular appointments with the pharmacist throughout the therapy are integrated for follow-up. The primary plan needs to be reevaluated and, if necessary, adjusted according to the patient’s needs. It can be described as comprehensive drug therapy management.
To detect potential DRPs and prevent or solve them, therapeutic outcome monitoring serves as a helpful tool [11]. Desired therapeutic outcomes, such as reduction of emetic episodes and degree of nausea, patient knowledge about a certain drug, compliance, etc., are selected to monitor outcome. Continuity can only be achieved with a thorough documentation of the patient-specific data. The medication record listing all drugs a patient is taking at one time gives an overview and helps in interpreting the patient’s situation. A number of problems can be detected just from analyzing the record. Not only the medication-related information should be collected, but also demographic data, information on life style (e.g., diet, exercise, social drug use), religious affiliations, and social background, should be recorded. This information allows a realistic picture of the patient and assessment of the situation. Fig. 2 shows the connection between the complex drug regimens of a cancer patient and the continuous pharmaceutical care process.
Target populations for pharmaceutical care
It seems that, in particular, patients with complex drug regimens or chronic diseases and those who frequently need to be hospitalized benefit from pharmaceutical care. These characteristics apply to many oncology patients. Anticancer therapy presents various desirable and undesirable outcomes. It is the main focus of the oncology care team to improve the desirable outcomes, such as cure of disease, slowing disease progression, decreasing symptoms, and reducing the incidence of undesirable outcomes such as mortality, disease progression, adverse effects, severe organ toxicity, and drug resistance. Some types of toxicity may be dose limiting and even lead to an interruption of the therapy. Thus, therapeutic success is strongly connected to the extent of therapy-associated toxicity. An efficient supportive care in order to control these ADRs is imperative for optimal treatment outcomes.
Pharmaceutical care of the cancer patient
Within anticancer therapy, drug regimens are administered following established protocols that have been generated in clinical trials and proven efficient for the respective indication. The administration of supportive medication is not as controlled as chemotherapy itself. Various settings emphasize supportive care in a different manner. Furthermore, in supportive care, evidence-based therapy is not as yet usual practice. Additionally, main parts of the supportive therapy are not carried out by the oncology clinic but by GPs or the patients themselves. This often leads to less effective protection of adverse drug events and thus a decreased quality of life. Therefore, supportive care strategies turn out to be a major field for the oncology pharmacist offering pharmaceutical care to the cancer patient. The two care approaches (supportive and pharmaceutical) work well together sharing common objectives.
Especially in ambulatory care, the continuous monitoring of the medication use process should be mandatory. Patients receiving care in the community often have to experience fragmented services. The prescriber often does not see the patient until the next visit in the clinic or outpatient department, which might be after a few weeks. In the meantime, ADRs can occur that may not be detected in time. Furthermore, patients tend to see more than one physician included in the cancer care process, as well as alternative practitioners, and they are exposed to a variety of OTC products. The different prescribers, nurses, and relatives as administrators, and the patients themselves with their self-medication, are all part of the individual drug therapy team. Thus, all need to be included in the collaborative process.
A group of British experts drew up a policy framework for commissioning cancer services. They suggest the establishment of structures that support the seamless care of cancer patients in the community setting in a network of all parties in order to make use of the respective specialty knowledge [23]. The information flow at discharge from hospital to home should be optimized utilizing pharmaceutical care plans to make sure that the efficient distribution of the medication to the patient is not interrupted.
A few examples on how pharmacists’ activities in supportive care expanded from the traditional tasks towards the patient-oriented tasks in the framework of pharmaceutical care are listed in Table 4. Within the pharmaceutical care process, the application of agreed therapeutic algorithms can be assured on an individual basis. Patient adherence can be improved by patient education before and during treatment cycles combined with patient counseling regarding drug therapy, adverse effects, and complementary treatment options.
Research
There are numerous publications on the philosophy and theoretical background of pharmaceutical care. Additionally, there are many reports on the implementation into practice settings. The major gap seems to be the lack of scientific evidence of the impact of pharmaceutical care on outcome. Kennie et al. addressed this problem and critically analyzed pharmaceutical care research literature in order to determine deficiencies in study design and research methods and to formulate recommendations to improve the situation [16]. Among other recommendations, they emphasize the importance of using the term “pharmaceutical care” properly. In the evaluated studies, they found that the term had been used to describe other pharmacy services, such as pharmacokinetic services or patient counseling, which on their own do not constitute pharmaceutical care. They also call for scientific standards such as controlled study designs that should be implemented. A further suggestion is the development of a pharmaceutical care network that can coordinate the international effort to improve the research and to identify fields of interest. The Pharmaceutical Care Network Europe (PCNE) has taken up these suggestions and meets annually to address these questions.
Mobach explains the traps of pharmaceutical care research (PCR) [20]. He describes it as a relatively new scientific field that needs to be defined and standards that need to be created. PCR ranges between natural science and social sciences. These directions have their own rules, which need to be linked and adapted to achieve valid results. It is science in the field rather than in the laboratory and thus much more exposed to a variety of environmental influences. For these conditions, specific rules are to be established through studies.
In oncology, so far there is little scientific evidence on the feasibility of pharmaceutical care and actual benefit to the patient. In Canada, projects have been carried out that suggest implementation of suitable outcome parameters to evaluate the impact of pharmaceutical services in oncology [6]. Data on outcome facilitate the discussion about the necessity of the offered services.
At the University of Bonn, Germany, two studies are currently being carried out that try to close this gap. These studies try to apply scientific standards to study design. Thoroughly worked out study protocols including informed consent, patient insurance, etc., were approved by the ethical committee. The pilot phase of the first project is almost completed and will be introduced briefly as an example. The study aims to evaluate the feasibility of pharmaceutical care in the ambulatory setting and the benefit to oncology patients. The design has been developed in close cooperation with biometricians. A prospective, controlled study design with a preceding control group was selected that approached the standards of clinical studies as closely as possible (Fig. 3).
Patients with breast or ovarian cancer undergoing chemotherapy are included either into a control or an intervention group. For the intervention group, the following key interventions were defined:
-
Regular appointments of the research pharmacist with the patients to define the patients’ needs and to detect and solve drug-related problems
-
Optimization of supportive care and application of the acquired therapeutic algorithms.
In order to measure outcome in both groups, the following instruments were chosen:
-
EORTC QLQ-C30 v3.0 questionnaire [1] and a visual analogue scale (VAS) to assess quality of life
-
A patient diary to document nausea and vomiting [10]
-
A patient satisfaction questionnaire referring to the given information [18].
Preliminary data of the pilot phase suggest an improvement of the measured outcomes. During chemotherapy, the quality of life decreased only 4 points (median) in the intervention group compared to 8 points in the control group. The median global patient satisfaction was 4.6 in the intervention group compared to 3.9 in the control group on a 5-point Likert scale. The severity of nausea and the frequency of emesis could be reduced.
The second project aims to develop pharmaceutical care for lung cancer patients, with special focus on the fatigue syndrome. This project has recently been initiated.
Integration in current health policy trends
Currently discussed care concepts, such as case- and disease-management programs (DMP), aim at improving patients’ outcomes by following evidence-based therapeutic guidelines, by trying to meet patients’ needs, and by taking into account economic aspects. All models advocate patients’ active participation in the therapeutic process and try to integrate quality assurance measures.
Todd et al. describe disease management (DM) in their review as a system [22]. They suggest that a common vision on what should be achieved is necessary to implement DM. All is based on the idea of continuous quality improvement. With the use of selected components such as databases, guidelines, outcome assessment, communication tools, etc., this aim is to be reached. Again, in this context, a consensus among all health care providers is seen as inevitable.
Certain cancers are considered to be chronic diseases. Thus, considerable effort is made to improve the overall treatment quality in every stage of the treatment process while reducing the according costs. In terms of drug therapy, DM aims to integrate the above-mentioned therapeutic goals in a standardized manner to obtain transparency for the patient and the third-party payers. It fosters interdisciplinary approaches in order to achieve these goals. This requires that pharmacists get more involved in the care of the patient.
Pharmaceutical care concepts seem to have a good potential of supporting the idea of DMPs. However, it is mandatory to document the impact of pharmaceutical care on patient outcomes in order to comply with the demand for transparency.
Conclusions
Cancer patients are a target population for pharmaceutical care due to particular information needs and a multitude of drug-related problems. Supportive medication plays a major role in developing these pharmaceutical care programs. The implementation of pharmaceutical care might also improve communication between health care professionals and support the idea of a multidisciplinary team approach. With the knowledge that has been accumulated within the last decade in the discipline of oncology pharmacy through implementation of different services for oncology patients, pharmacists are increasingly prepared to offer pharmaceutical care for cancer patients. The benefit of pharmaceutical care programs has to be evaluated in controlled trials. Preliminary results suggest especially an increased patient satisfaction with cancer treatment education.
Pharmaceutical care concepts support the idea of supportive care and may be integrated in DMPs. All concepts discussed have the same objectives in common: They aim to improve patient care and thus patients’ quality of life by making use of according strategies. It appears reasonable to combine those concepts to achieve a maximum benefit for the individual patient and consequently for the whole health system.
References
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Alliance for Aging Research (1998) When Medication Hurts Instead of Helps: Preventing Medication Problems in Older People. Washington D.C.
American Federation of Clinical Oncologic Societies (1998) Access to quality cancer care: consensus statement. J Clin Oncol 16:1628–1630
American Society of Health-System Pharmacists (1996) ASHP guidelines on a standardized method for pharmaceutical care. Am J Health Syst Pharm 53:1713–1716
Berard CM, Mahoney CD (1995). Cost-reducing treatment algorithms for antineoplastic drug-induced nausea and vomiting. Am J Health Syst Pharm 52:1879–1885
Broadfield L (1995) Pharmaceutical care in oncology pharmacy practice: A method for using outcome indicators. J Oncol Pharm Pract 1:9–14
Cipolle RJ, Strand LM, Morley PC (1998) Pharmaceutical Care Practice. McGraw-Hill, New York Chapter 3:73–120
Dranitsaris G, Leung P, Warr D (2001) Implementing evidence based antiemetic guidelines in the oncology setting: results of a 4-month prospective intervention study. Support Care Cancer 9:611–618
Engstrom C, Hernandez I, Haywood J, Lilenbaum R (1999) The efficacy and cost effectiveness of new antiemetic guidelines. Oncol Nurs Forum 26:1453–1458
Freidank A (1999) Schemata zur Prophylaxe von Zytostatika-induzierter Emesis und Nausea. Krankenhauspharmazie 20:49–54
Hepler CD (1997) Pharmaceutical Care and Therapeutic Outcomes Monitoring. J Appl Therap 1:285–294
Hepler CD, Strand LM (1990). Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 47:533–543
Hoare D, Beer C (1995) Guidelines for the pharmaceutical care of cancer patients. Pharm J 255:841–842
Hon YY, Evans WE (1998) Making TDM work to optimize cancer chemotherapy: a multidisciplinary team approach. Clin Chem 44:388–400
Inhaltskatalog Onkologische Pharmazie (2000). http://www.ifahs.org/apotheker/inhaltskatalog.html
Kennie NR, Schuster BG, Einarson TR (1998) Critical Analysis of the Pharmaceutical Care Research Literature. Ann Pharmacother 32:17–26
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of Adverse Drug Reactions in Hospitalized Patients. JAMA 279:1200–1205
Liekweg A, Eckhardt M, Taylor SCM, Erdfelder E, Jaehde U (2003). Patient satisfaction with information on cancer treatment – A useful outcome measure for pharmaceutical care? Pharm World Sci, submitted for publication
Lindley CM (1994). Pharmaceutical Care: The Chemotherapy Patient. US Pharmacist 56–78.
Mobach MP (2001) From the laboratory to pharmaceutical care research—Part I. Pharm World Sci 23:205–209
Quality Standards for the Oncology Pharmacy Service (2001) 3rd ed. Onkopress, Oldenburg, Germany
Todd WE, Eichert JH, Toscani MR (1997) Disease Management—Building a Solid Foundation. Dis Manage Health Outcomes 1:26–31
Working Party Report (1997) Pharmaceutical care of cancer patients in the community. Pharm J 258:54–58
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented as an invited lecture at the 15th International Symposium Supportive Care in Cancer, Berlin, Germany, June 18–21, 2003
Rights and permissions
About this article
Cite this article
Liekweg, A., Westfeld, M. & Jaehde, U. From oncology pharmacy to pharmaceutical care: new contributions to multidisciplinary cancer care. Support Care Cancer 12, 73–79 (2004). https://doi.org/10.1007/s00520-003-0539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-003-0539-4